Abstract
1. Adaptation to a high-contrast sine-wave grating has been shown previously by Blakemore & Campbell (1969) to raise the modulation required to detect a low-contrast grating that has the same or similar spatial frequency as the adapting grating.
2. A similar adaptation effect occurs when the adaptation and test gratings are seen binocularly and are presented off the plane of fixation. When the gratings are not located on the plane of fixation, however, the greatest rise in threshold following adaptation occurs for test gratings presented in the same plane as the adapting grating. Thus, the neural mechanisms adapted to the high contrast patterns must be processing disparity information.
3. The spatial frequency response of the disparity adaptation effect has been measured by adapting to gratings of different spatial frequencies presented at a given disparity, and comparing threshold elevations for identical test gratings presented in the same (disparate) plane as the adapting grating or in the plane of fixation.
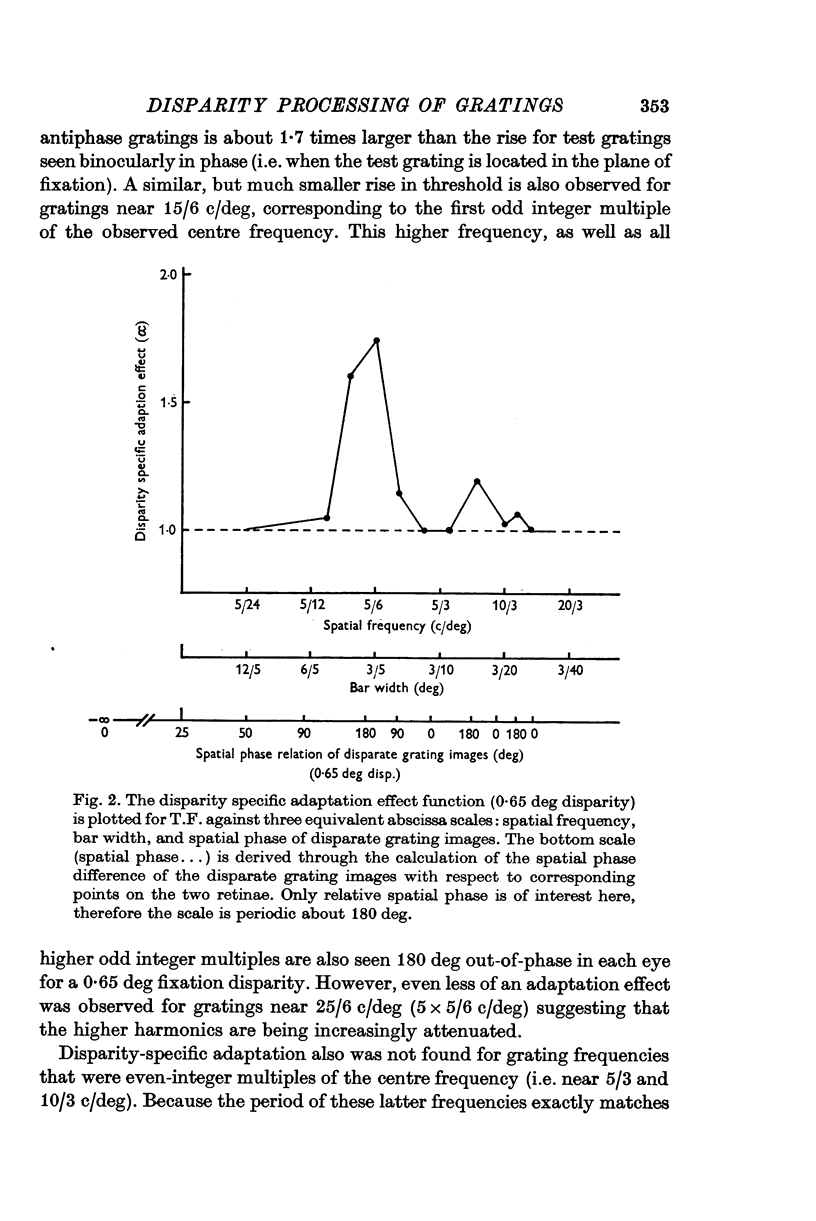
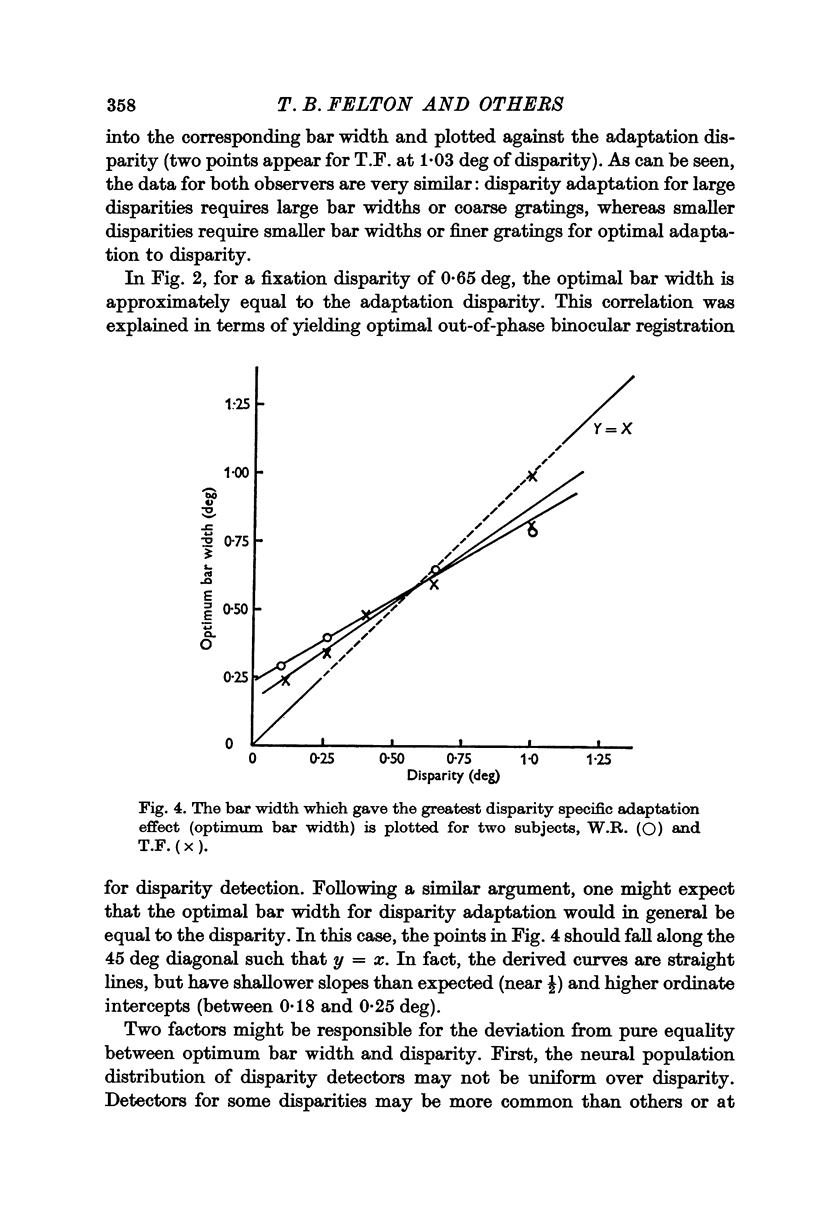
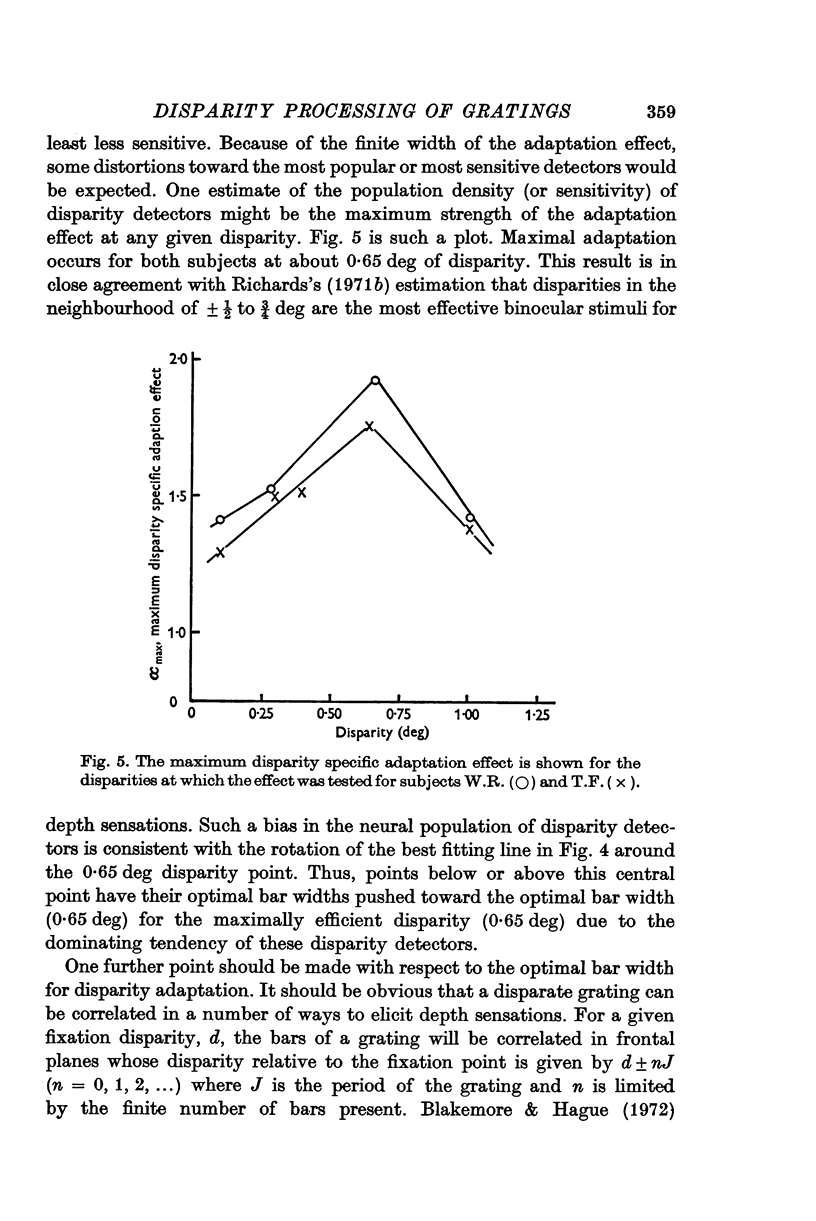
4. The unbiased adaptation effect specific to disparity is greatest for gratings whose periods are twice the disparity.
5. There is no adaptation effect specific to disparity for individuals possessing only convergent or only divergent disparity mechanisms.
6. The results suggest that disparity mechanisms make bar by bar correlations as opposed to edge by edge correlations and that narrow bar detectors feed small disparity mechanisms whereas wide bar detectors feed large disparity mechanisms.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Hague B. Evidence for disparity detecting neurones in the human visual system. J Physiol. 1972 Sep;225(2):437–455. doi: 10.1113/jphysiol.1972.sp009948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Julesz B. Stereoscopic depth aftereffect produced without monocular cues. Science. 1971 Jan 22;171(3968):286–288. doi: 10.1126/science.171.3968.286. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Richards W. Stereopsis and stereoblindness. Exp Brain Res. 1970;10(4):380–388. doi: 10.1007/BF02324765. [DOI] [PubMed] [Google Scholar]
- Sachs M. B., Nachmias J., Robson J. G. Spatial-frequency channels in human vision. J Opt Soc Am. 1971 Sep;61(9):1176–1186. doi: 10.1364/josa.61.001176. [DOI] [PubMed] [Google Scholar]


