Abstract
1. The results described are for detailed analyses of fifty-four isolated LGN units, in response to monochromatic stimuli presented against achromatic, mid-mesopic backgrounds. Forty-seven were positively identified cells from the A-laminae; the remaining seven were fibres from the optic radiation.
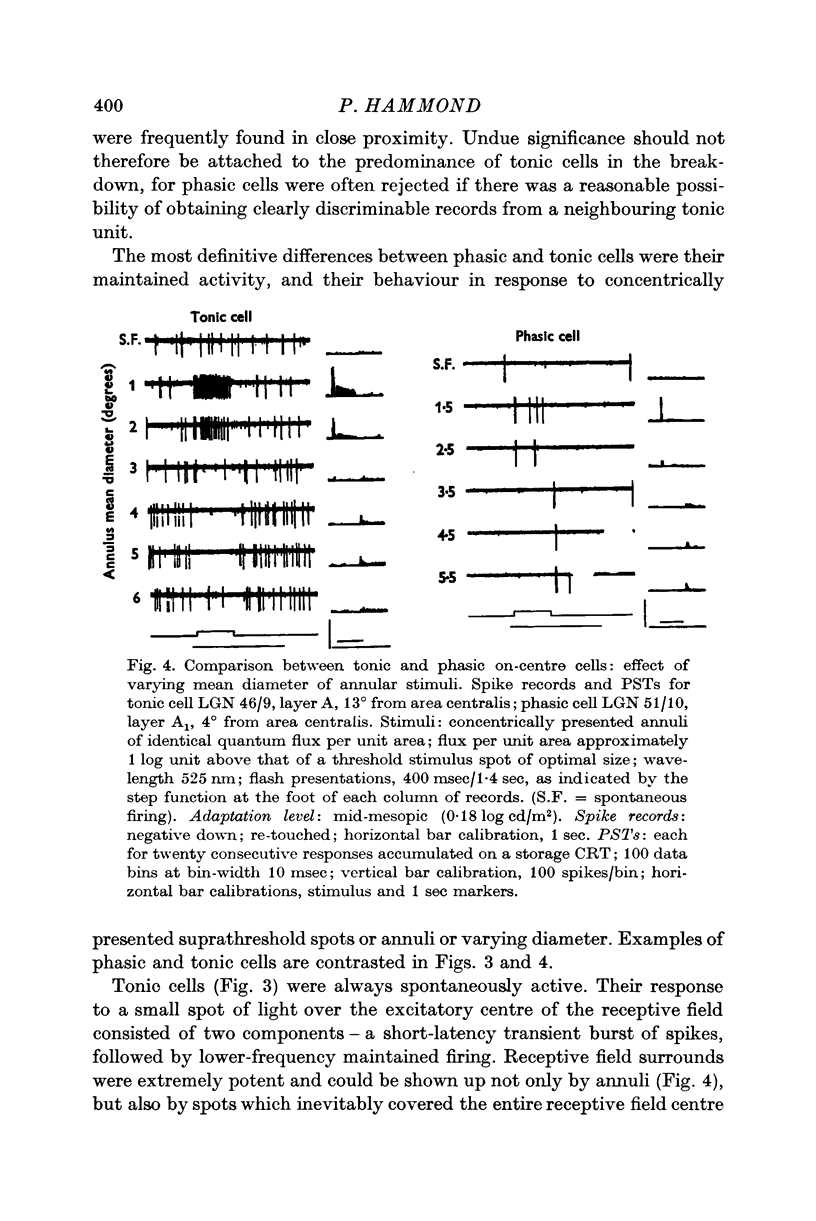
2. Cells are classified according to firing pattern. Phasic cells respond almost exclusively with a discharge transient. Tonic cells, by contrast, give a maintained component in addition. In general, tonic cells possess higher spontaneous firing frequencies than phasic cells and the antagonistic surrounds of their receptive fields are more potent. In other respects the two classes appear to be functionally similar.
3. All cells within the A-laminae receive input involving both rods and 556 nm cones.
4. The spatial organization of geniculate receptive fields, unlike retinal fields, is little different for cone and rod vision. In the infrequent instances where a change is apparent, it is small and can go in either direction: rod fields are then on balance slightly larger than cone fields.
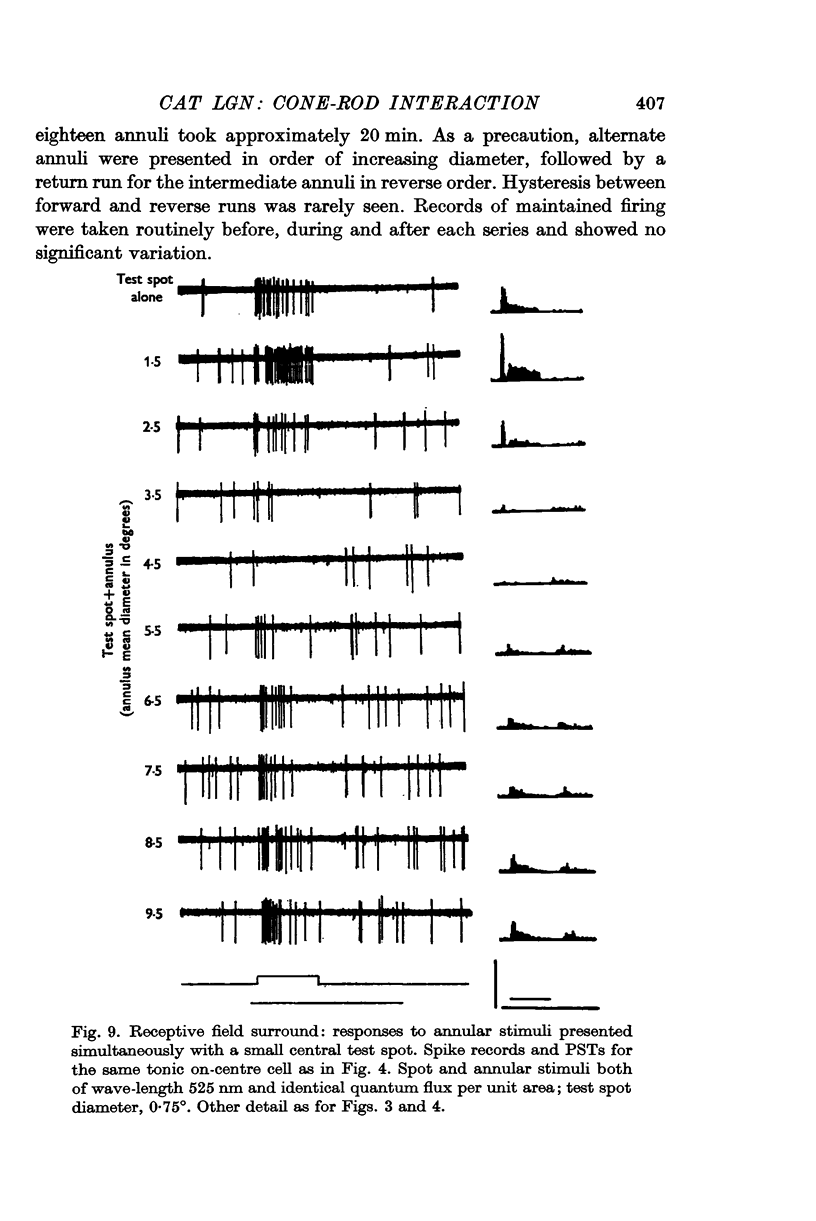
5. The locus of maximum sensitivity for the receptive field surround is described by a circle, concentric with the field centre; it is invariant with respect to stimulus geometry, or changeover from cone to rod vision.
6. This result implies that the receptive field surround mechanism does not extend through the field centre. It supports the notion that the centre and surround of each geniculate cell receptive field are mediated by discrete retinal inputs.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The interpretation of the extracellular response of single lateral geniculate cells. J Physiol. 1962 Aug;162:451–472. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971 Jun 9;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARTNALL H. J. A. The interpretation of spectral sensitivity curves. Br Med Bull. 1953;9(1):24–30. doi: 10.1093/oxfordjournals.bmb.a074302. [DOI] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Gouras P., Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. J Physiol. 1966 May;184(2):499–510. doi: 10.1113/jphysiol.1966.sp007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. The effects of light-adaptation on rod and cone receptive field organization of monkey ganglion cells. J Physiol. 1967 Oct;192(3):747–760. doi: 10.1113/jphysiol.1967.sp008328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Chromatic sensitivity and spatial organization of cat visual cortical cells: cone-rod interaction. J Physiol. 1971 Mar;213(2):475–494. doi: 10.1113/jphysiol.1971.sp009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., James C. R. The Purkinje shift in cat: extent of the mesopic range. J Physiol. 1971 Jul;216(1):99–109. doi: 10.1113/jphysiol.1971.sp009511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Spatial organization of receptive fields of LGN neurones. J Physiol. 1972 Apr;222(1):53P–54P. [PubMed] [Google Scholar]
- Hammond P. Spectral properties of dark-adapted retinal ganglion cells in the plaice (Pleuronectes platessa, L.). J Physiol. 1968 Apr;195(3):535–556. doi: 10.1113/jphysiol.1968.sp008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Local adaptation in cat LGN cells: evidence for a surround antagonism. Vision Res. 1971 Jun;11(6):501–509. doi: 10.1016/0042-6989(71)90073-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Darian-Smith I., Bishop P. O. Binocular corresponding receptive fields of single units in the cat dorsal lateral geniculate nucleus. Vision Res. 1969 Oct;9(10):1297–1303. doi: 10.1016/0042-6989(69)90117-5. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]


