Abstract
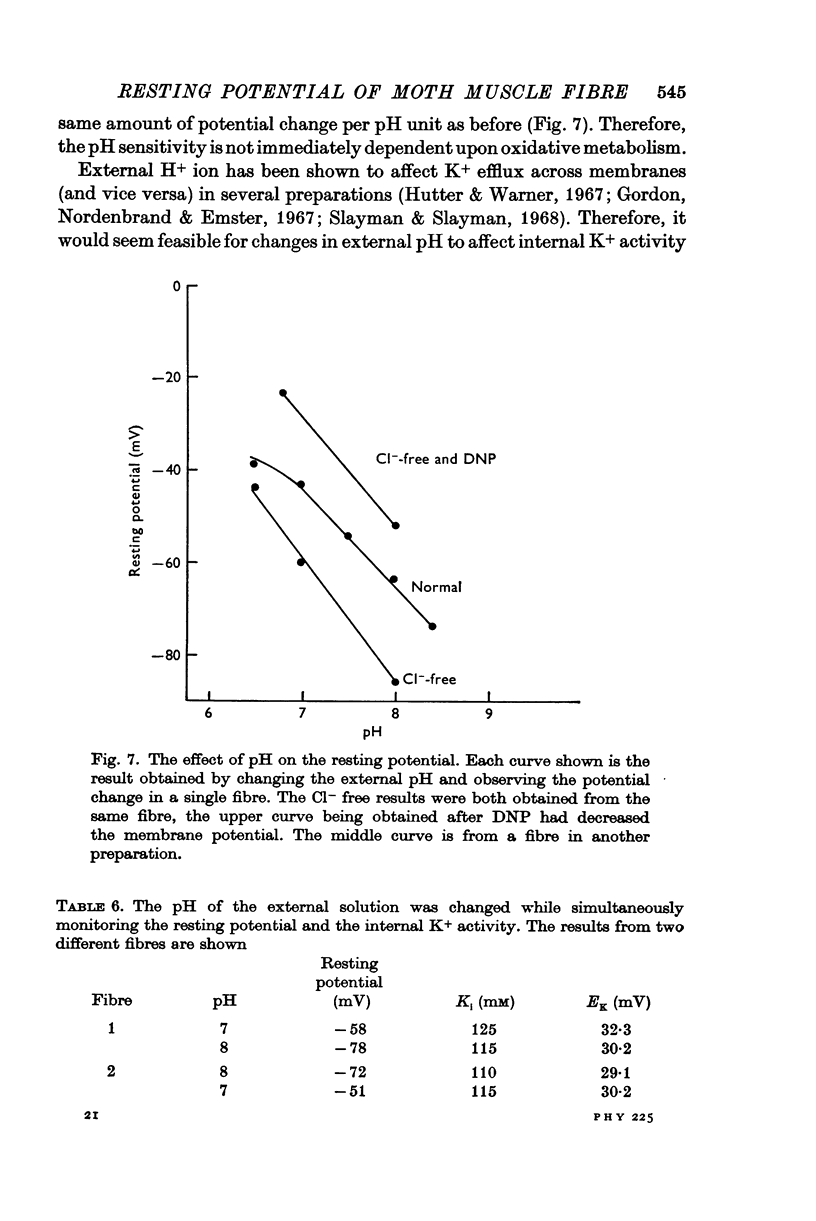
1. The membrane of the moth muscle fibre was tested for resting permeability to various ions: it is not permeable to Mg2+ or Ca2+; it is slightly permeable to Na+ and NH4+; it is appreciably permeable to Cl-, but Cl- is passively distributed; it is apparently permeable to H+ but effects of HCO3- are not ruled out; and it is primarily permeable to K+.
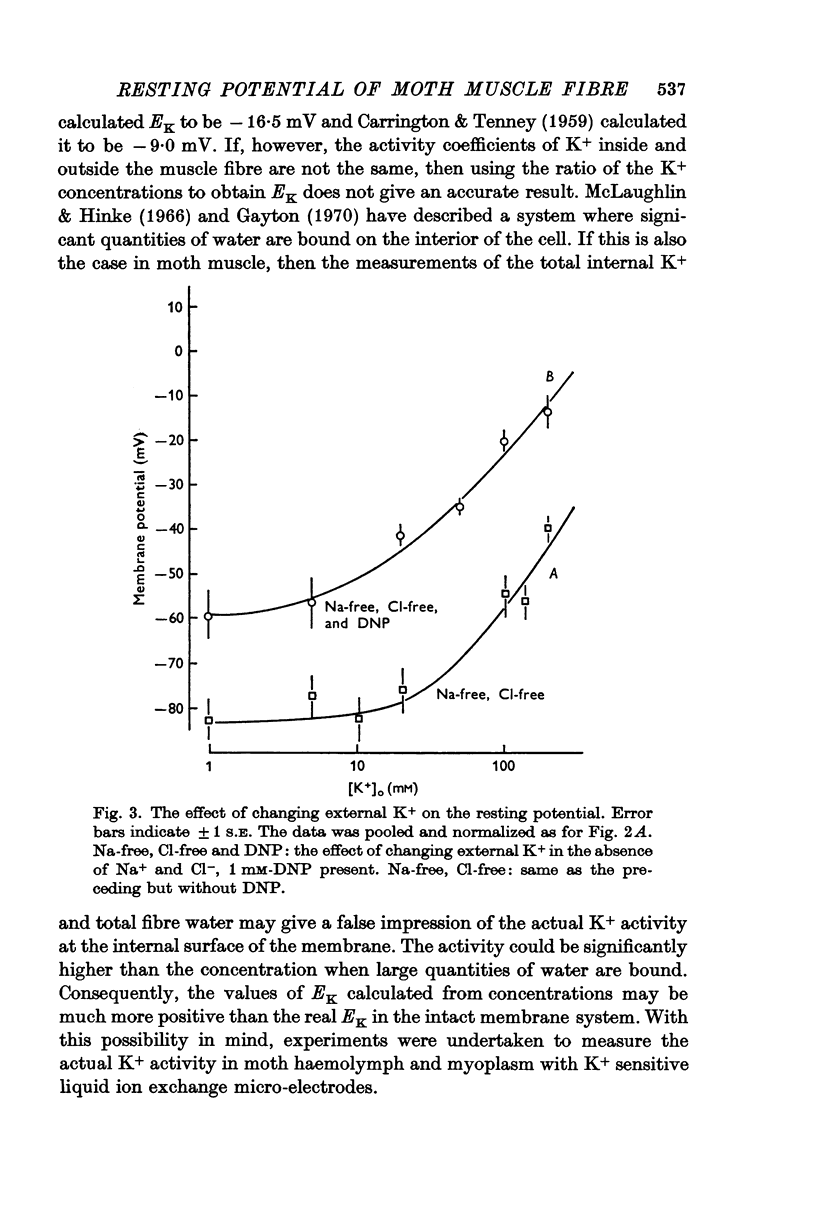
2. Measurement of the internal K+ activity showed that EK is less negative than the resting potential.
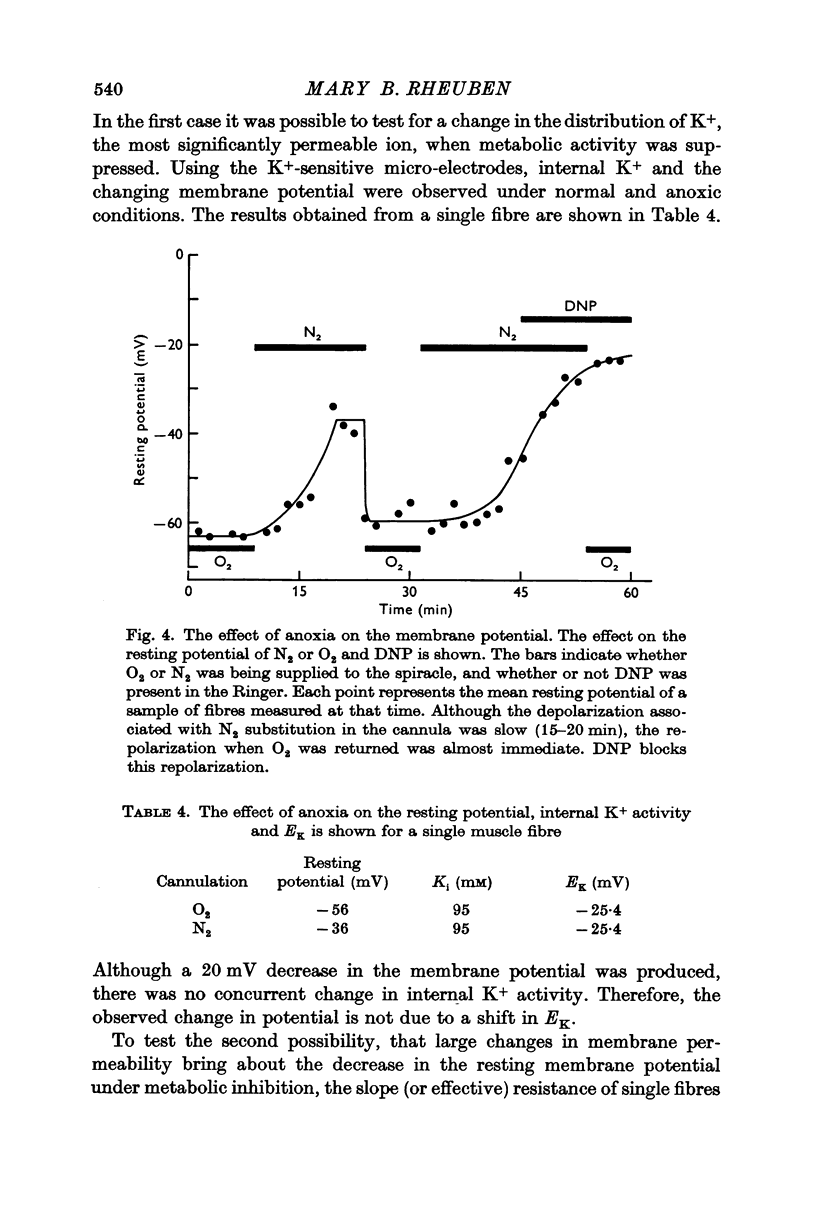
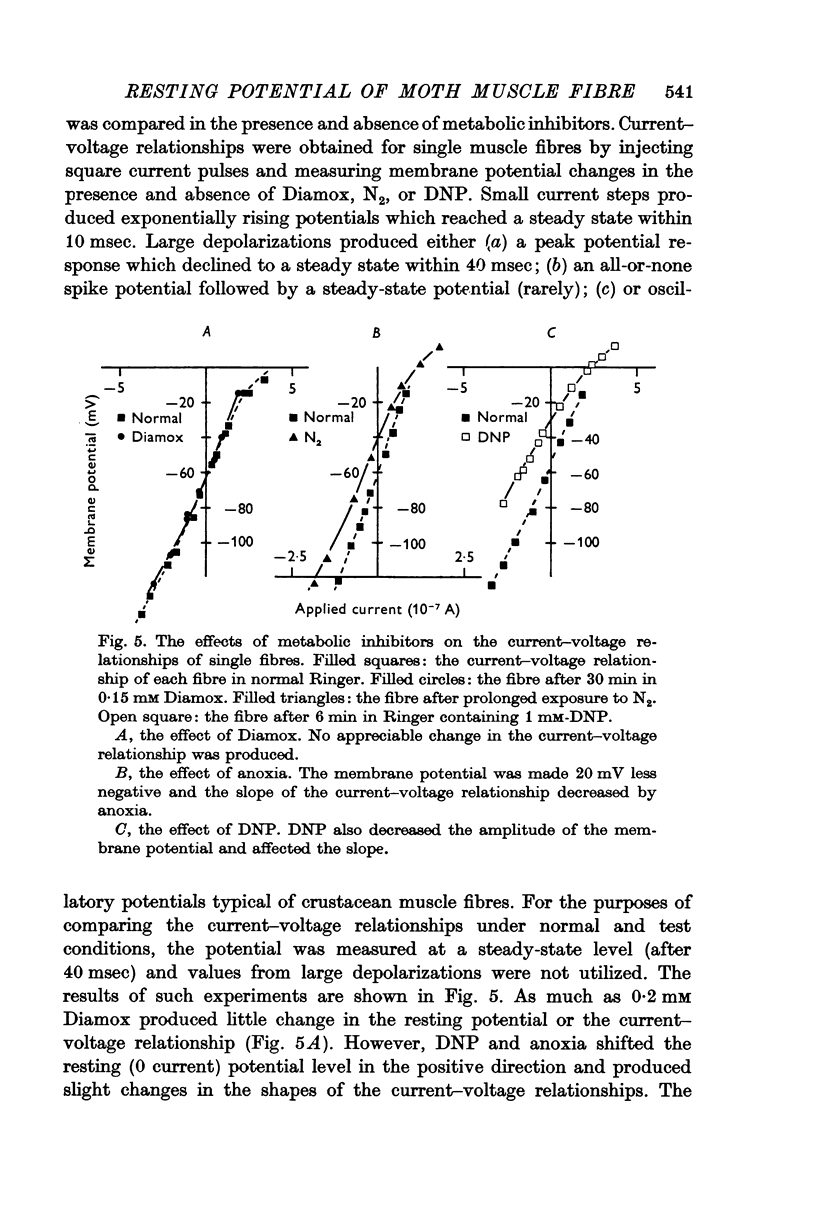
3. In the presence of DNP, or under anoxia, the membrane potential approaches, EK; there is a small concomitant decrease in effective membrane resistance.
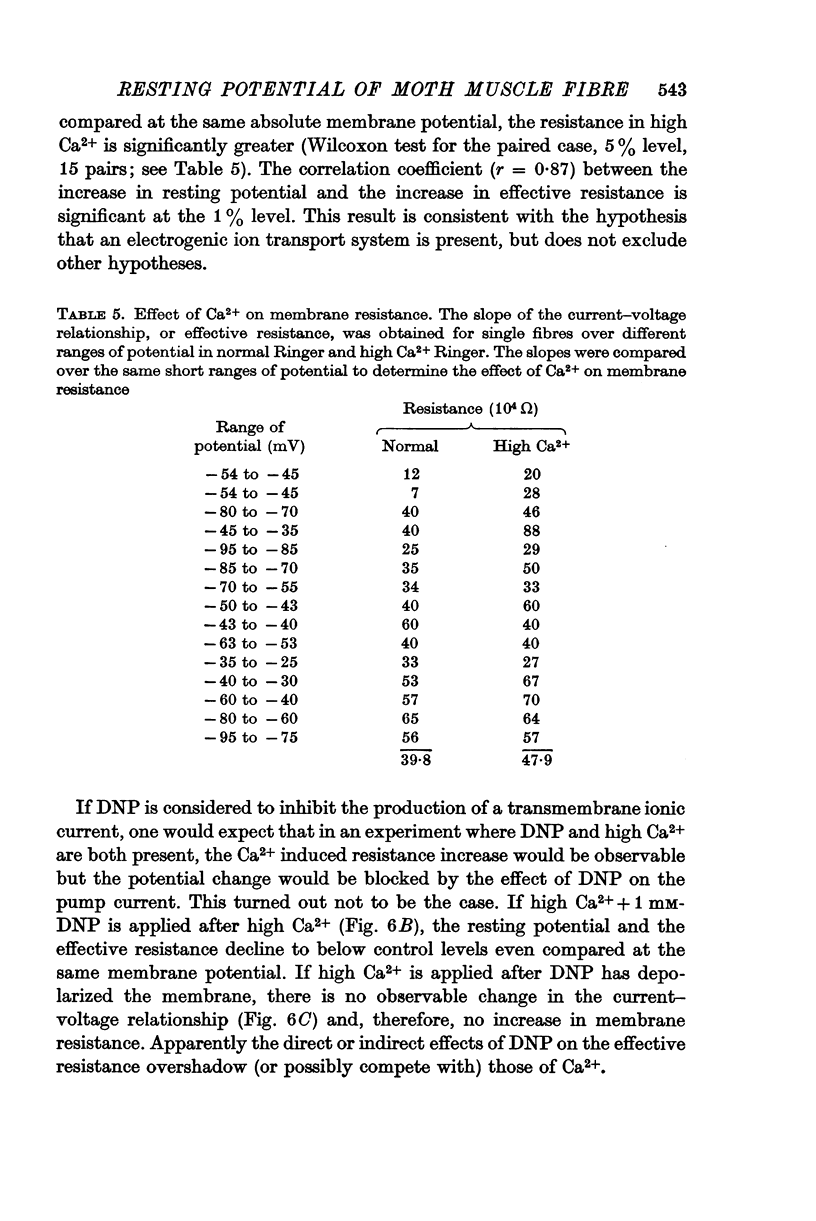
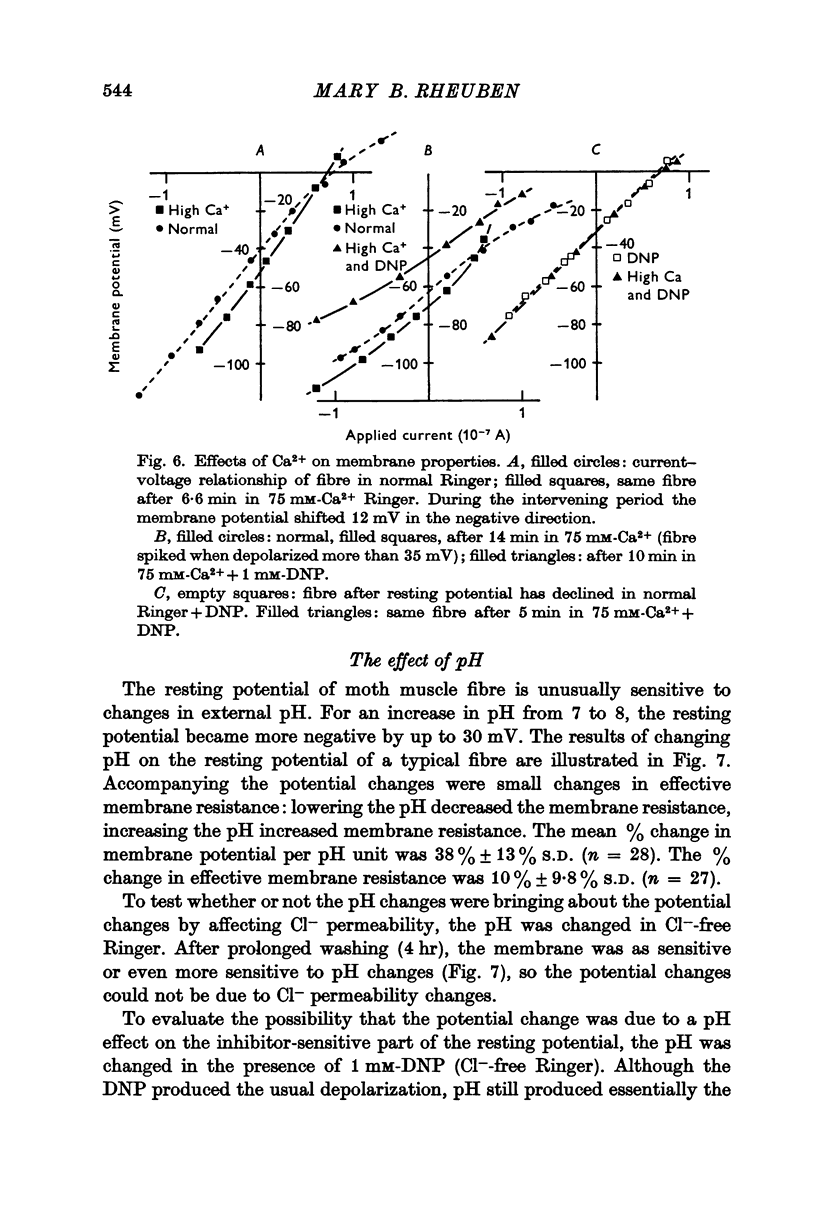
4. An increase in external Ca2+ concentration is accompanied by increased effective membrane resistance and an increase in amplitude of the negative resting potential.
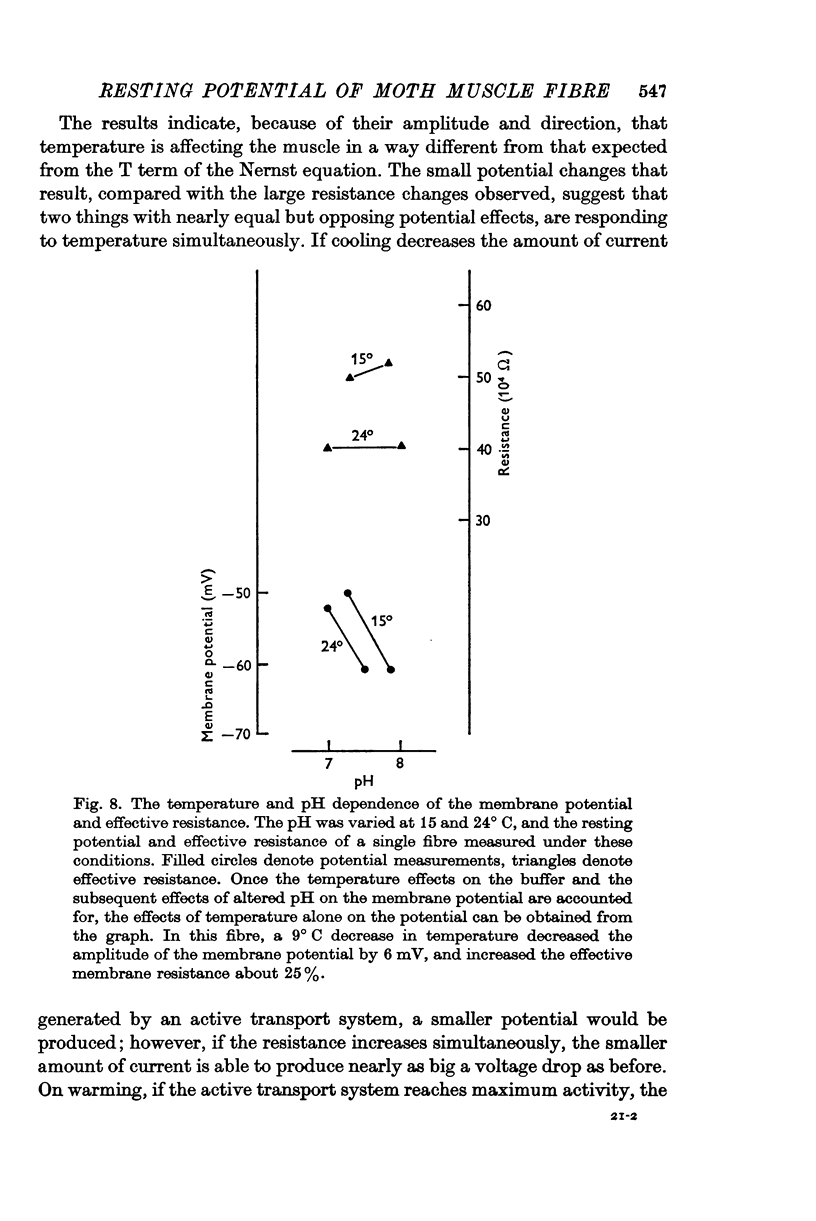
5. Cooling the membrane (below room temperature) decreased the amplitude of the resting potential by 4-16 mV per 10° C, and was accompanied by a large increase in effective membrane resistance.
6. The experimental results most readily fit the hypothesis that the resting potential of the moth muscle fibre, although the membrane is highly permeable to K+, Cl- and apparently to H+, is primarily maintained by an electrogenic transport process which generates an ionic current across the membrane. The possibility that the concentration gradient of H+ ions is metabolically maintained at a level sufficient to explain the resting potential was considered to be unlikely but could not be directly excluded.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Lecar H. Ammonium ion currents in the squid giant axon. J Gen Physiol. 1969 Mar;53(3):342–361. doi: 10.1085/jgp.53.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward P. K. Response characteristics of muscle afferents in the domestic duck. J Physiol. 1970 Nov;211(1):1–17. doi: 10.1113/jphysiol.1970.sp009262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D. A ouabain-sensitive membrane conductance. J Physiol. 1968 Feb;194(2):521–533. doi: 10.1113/jphysiol.1968.sp008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A. Action potential of insect muscle examined with intra-cellular electrode. Jpn J Physiol. 1954 Mar 5;4(1):65–78. doi: 10.2170/jjphysiol.4.65. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G. The effects of some common cations on neuromuscular transmission in insects. J Physiol. 1955 Jan 28;127(1):90–103. doi: 10.1113/jphysiol.1955.sp005240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Gruener R., Hayashi H., Sakata H., Grinnell A. D. Effect of external and internal pH changes on K and Cl conductances in the muscle fiber membrane of a giant barnacle. J Gen Physiol. 1968 Nov;52(5):773–792. doi: 10.1085/jgp.52.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart H. Ionic composition of haemolymph and myoplasm in lepidoptera in relation to their membrane potentials. Arch Int Physiol Biochim. 1966 Sep;74(4):603–613. doi: 10.3109/13813456609059939. [DOI] [PubMed] [Google Scholar]
- Huddart H. The effect of potassium ions on resting and action potentials in lepidopteran muscle. Comp Biochem Physiol. 1966 May;18(1):131–140. doi: 10.1016/0010-406x(66)90337-9. [DOI] [PubMed] [Google Scholar]
- Huddart H., Wood D. W. The effect of DNP on the resting potential and ionic content of some insect skeletal muscle fibres. Comp Biochem Physiol. 1966 Aug;18(4):681–688. doi: 10.1016/0010-406x(66)90204-0. [DOI] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Sodium and water binding in single striated muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1966 Sep;44(5):837–848. doi: 10.1139/y66-102. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft J. P. Effects of some inhibitors on the temperature-dependent component of resting potential in lobster axon. J Gen Physiol. 1967 Aug;50(7):1835–1848. doi: 10.1085/jgp.50.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood P. N. Insect neuromuscular mechanisms. Am Zool. 1967 Aug;7(3):553–582. doi: 10.1093/icb/7.3.553. [DOI] [PubMed] [Google Scholar]
- WERMAN R., McCANN F. V., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. I. The effects of alkali-earth cations on the neuromuscular system of Romalea microptera. J Gen Physiol. 1961 May;44:979–995. doi: 10.1085/jgp.44.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD D. W. The effect of ions upon neuromuscular transmission in a herbivorous insect. J Physiol. 1957 Aug 29;138(1):119–139. doi: 10.1113/jphysiol.1957.sp005841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. L., Jr, Brown A. M. Unified account of the variable effects of carbon dioxide on nerve cells. Science. 1970 Mar 13;167(3924):1502–1504. doi: 10.1126/science.167.3924.1502. [DOI] [PubMed] [Google Scholar]
- Wareham A. C., Duncan C. J., Bowler K. Permeability and excitation of insect muscle. Nature. 1968 Mar 9;217(5132):970–972. doi: 10.1038/217970b0. [DOI] [PubMed] [Google Scholar]
- den Hertog A., Ritchie J. M. A comparison of the effect of temperature, metabolic inhibitors and of ouabain on the electrogenic componen of the sodium pump in mammalian non-myelinated nerve fibres. J Physiol. 1969 Oct;204(3):523–538. doi: 10.1113/jphysiol.1969.sp008929. [DOI] [PMC free article] [PubMed] [Google Scholar]


