Abstract
1. A study has made of the effect of (Na+-K+-Mg2+)-activated membrane ATP-ase inhibitors on the acetylcholine release from the terminals of enteric nerves and from cortical slices.
2. The resting output of acetylcholine from slices of rat cortex was not affected by tetrodotoxin or by noradrenaline, indicating the lack of propagated activity during rest. Furthermore, there was an output of acetylcholine in the absence of Ca.
3. The resting acetylcholine output from cortical slices was increased by (a) addition of ouabain or (b) administration of sodium p-hydroxymercuribenzoate (PHMB), (c) sodium withdrawal and (d) Ca replacement by Ba+.
4. Omission of Ca in the presence of 1 mM ethyleneglycol-bis-(β-aminoethyl-ether)-N,N′-tetra-acetic acid (EGTA) did not affect the increase of acetylcholine release by the inhibition of (Na+-K+-Mg2+)-activated ATP-ase induced by ouabain or by PHMB, but reduced that due to Na removal.
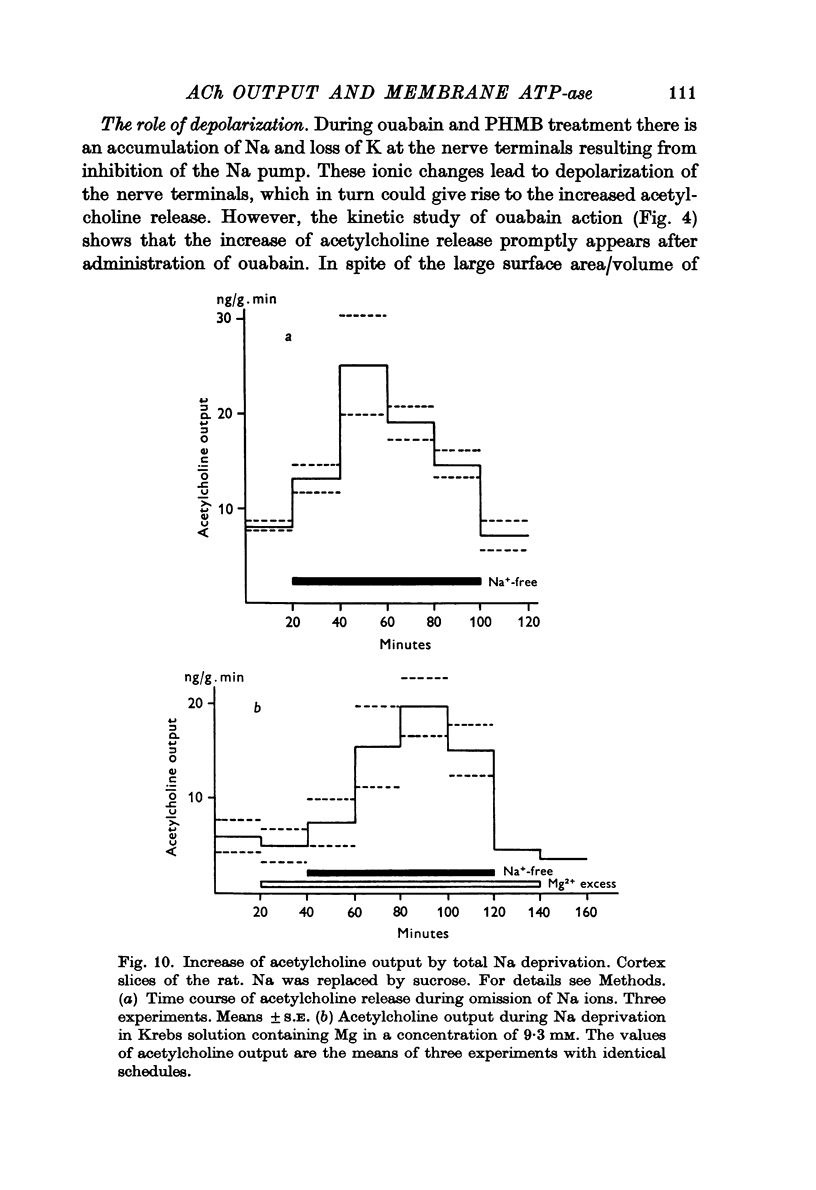
5. Ouabain increased acetylcholine release promptly.
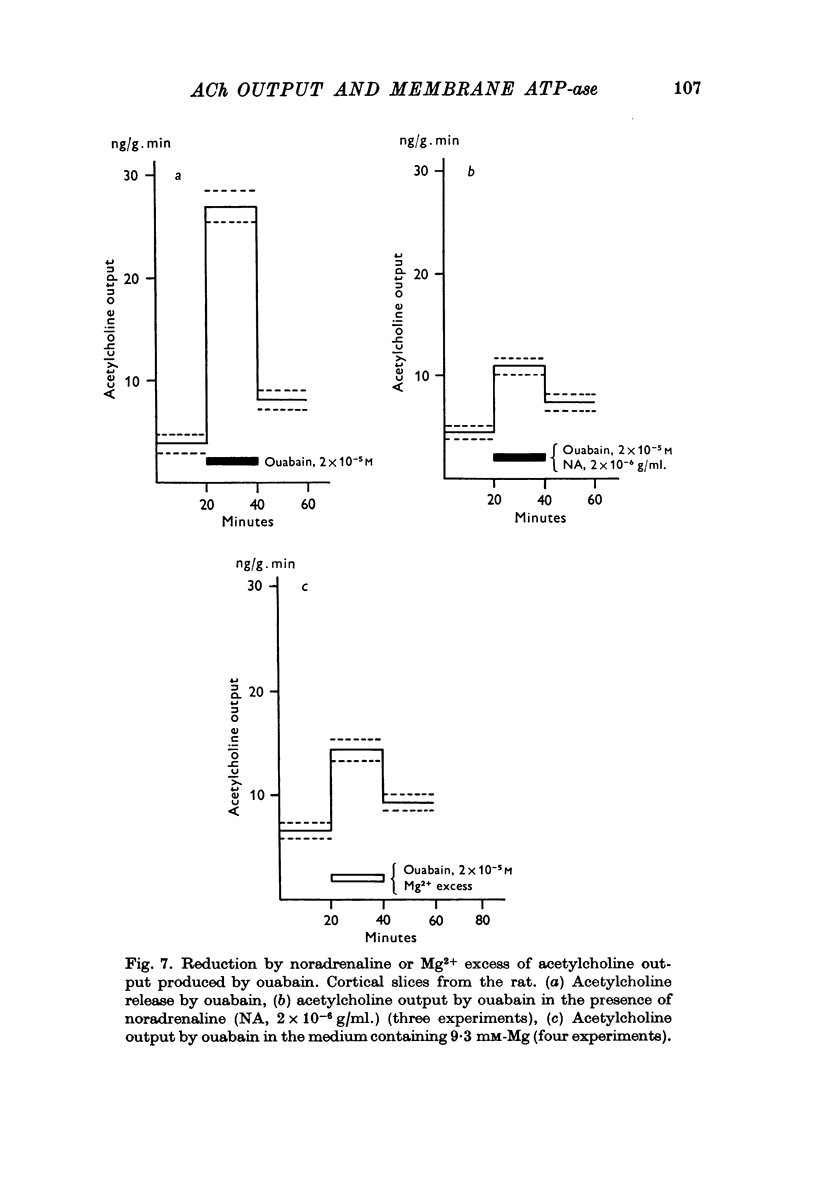
6. Mg-excess (9·3 mM), noradrenaline and adrenaline were capable of reducing the increase of acetylcholine release from cortical slices evoked by ouabain, PHMB or by Ca replacement by Ba, but not by Na removal.
7. A possible role for (Na+-K+-Mg2+)-activated ATP-ase in the release of acetylcholine is discussed. It is suggested that the effect of Ca and Mg ions on acetylcholine release might be attributed to their ability to inhibit and activate the membrane ATP-ase, respectively.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R. I. THE ROLE OF SODIUM IONS IN THE METABOLISM OF ACETYLCHOLINE. Can J Biochem Physiol. 1963 Dec;41:2573–2597. [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Two phases of calcium entry during the action potential in giant axons of Loligo. J Physiol. 1970 Jun;208(2):80P–82P. [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Wiesmann W. P. Effect of sodium ions on calcium movements in isolated synaptic terminals. Proc Natl Acad Sci U S A. 1970 Jul;66(3):664–671. doi: 10.1073/pnas.66.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers M. B., Jr Factors influencing maintenance and release of acetylcholine in rat cortical brain slices. Int J Neuropharmacol. 1967 Sep;6(5):399–403. doi: 10.1016/0028-3908(67)90031-7. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Feldberg W. The action of potassium on the superior cervical ganglion of the cat. J Physiol. 1936 Mar 9;86(3):290–305. doi: 10.1113/jphysiol.1936.sp003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie A. L., Kosterlitz H. W., Watt A. J. Mode of action of morphine-like drugs on autonomic neuro-effectors. Nature. 1968 Dec 7;220(5171):1040–1042. doi: 10.1038/2201040a0. [DOI] [PubMed] [Google Scholar]
- Creese R., Taylor D. B. Entry of labeled carbachol in brain slices of the rat and the action of d-tubocurarine and strychnine. J Pharmacol Exp Ther. 1967 Aug;157(2):406–419. [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Knoll J., Vizi E. S. Effect of frequency of stimulation on the inhibition by noradrenaline of the acetylcholine output from parasympathetic nerve terminals. Br J Pharmacol. 1971 Jun;42(2):263–272. doi: 10.1111/j.1476-5381.1971.tb07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J., Vizi E. S. Presynaptic inhibition of acetylcholine release by endogenous and exogenous noradrenaline at high rate of stimulation. Br J Pharmacol. 1970 Nov;40(3):554P–555P. [PMC free article] [PubMed] [Google Scholar]
- Lloyd S., Pickford M. The effect of oxytocin and adrenaline on blood flow in the hind limb of the dog following chronic lumbar sympathectomy. J Physiol. 1967 Sep;192(1):43–52. doi: 10.1113/jphysiol.1967.sp008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann P. J., Tennenbaum M., Quastel J. H. Acetylcholine metabolism in the central nervous system: The effects of potassium and other cations on acetylcholine liberation. Biochem J. 1939 May;33(5):822–835. doi: 10.1042/bj0330822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Quastel J. H. Tetrodotoxin-sensitive uptake of ions and water byslices of rat brain in vitro. Biochem J. 1970 Nov;120(1):37–47. doi: 10.1042/bj1200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak R. L., Meeuws M. M. The influence of atropine on the release and uptake of acetylcholine by the isolated cerebral cortex of the rat. Biochem Pharmacol. 1966 Jul;15(7):989–992. doi: 10.1016/0006-2952(66)90176-6. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- SOMOGYI J., VINCZE I. Mitochondrial and extramitochondrial adenosine triphosphatase in brain tissue. II. Some properties of the extramitochondrial adenosine triphosphatase. Acta Physiol Acad Sci Hung. 1962;21:29–41. [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Somogyi J. Ueber die bindung der Ca-ionen an das Na+-K+-aktivierbare Adenosintriphosphatase-System des Gehirns. Experientia. 1964 Jan 15;20(1):28–29. doi: 10.1007/BF02146025. [DOI] [PubMed] [Google Scholar]
- Stahl W. L., Swanson P. D. Uptake of calcium by subcellular fractions isolated from ouabain-treated cerebral tissues. J Neurochem. 1969 Dec;16(12):1553–1563. doi: 10.1111/j.1471-4159.1969.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Swanson P. D. Effects of ouabain on acid-soluble phosphated and electrolytes of isolated cerebral tissues in presence or absence of calcium. J Neurochem. 1968 Feb;15(2):57–67. doi: 10.1111/j.1471-4159.1968.tb06175.x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Knoll J. The effects of sympathetic nerve stimulation and guanethidine on parasympathetic neuroeffector transmission; the inhibition of acetylcholine release. J Pharm Pharmacol. 1971 Dec;23(12):918–925. doi: 10.1111/j.2042-7158.1971.tb09893.x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. The inhibitory action of noradrenaline and adrenaline on release of acetylcholine from guinea-pig ileum longitudinal strips. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(2):199–200. doi: 10.1007/BF00537789. [DOI] [PubMed] [Google Scholar]


