Abstract
1. Micro-electrode recordings were obtained from over 100 single fibres in the cochlear nerve of the pentobarbitone or urethane anaesthetized guinea-pig. The acoustic system was calibrated at the tympanic membrane and threshold sound level measurements so corrected.
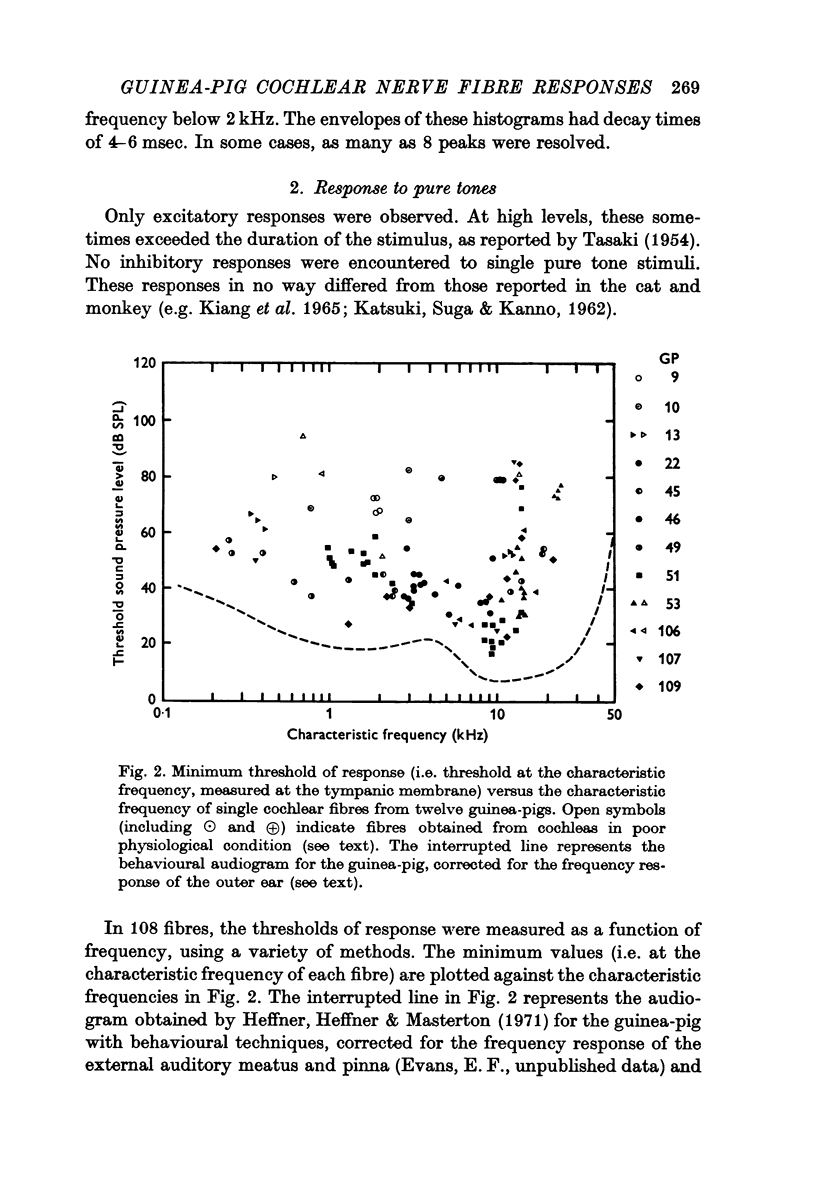
2. The minimum thresholds of the fibres approached with 10-20 dB of the behavioural thresholds reported in the literature. Exceptions to this were fibres from preparations where there was evidence of malfunction of the cochlea either from abnormally low perfusion or local damage, and a few high frequency fibres. With these high threshold fibres excepted, the range of thresholds at a given frequency in any one animal was less than 20 dB.
3. The slopes of the low and high frequency cut-offs of the frequency—threshold curves (`tuning curves') within 25 dB of minimum threshold, ranged from 10 to 60 and from 20 to 125 dB/octave respectively for fibres with characteristic frequencies below 2 kHz, increasing to 90-180 and 200-600 dB/octave respectively for fibres with characteristic frequencies at about 8 kHz. These slopes represent the minimum values for the high-frequency cut-offs, which increase towards 1000 dB per octave in some cases at higher levels above threshold. At 30-50 dB above threshold, the low frequency cut-offs become suddenly less steep and approximate to 5 dB per octave.
4. The relative sharpness of the frequency—threshold curves, measured as the `Q10 dB', i.e. the ratio of characteristic frequency to the band width at 10 dB above minimum threshold, ranged from 1 to 4 for fibres with characteristic frequencies below 2 kHz, to 3-15 for fibres with characteristic frequencies near 10 kHz.
5. The slopes and `Q10 dB' measures of the frequency—threshold curves of most of the abnormally high threshold fibres approximated to, or were lower than those of analogous measurements of the guinea-pig basilar membrane vibration patterns.
6. Four fifths of the cochlear nerve fibres had spontaneous discharge rates greater than 1/sec. No consistent relationship was observed between the rate of this activity and response properties, with the exception that nearly half of the high threshold fibres were silent. In these and other respects the response properties to tonal and click stimuli resembled those of cochlear nerve fibres in the cat. In no case was inhibition of the spontaneous discharge by single tones observed.
7. It is concluded that, contrary to earlier reports, the cochlear nerve fibres of the guinea-pig are substantially more frequency selective than the existing measurements of the guinea-pig basilar membrane displacement. In terms of band width, this discrepancy approaches a factor of ten. The finding of a considerable range of band widths within optimal preparations, and frequency—threshold curves approximating to the mechanical functions in fibres from pathological cochleas, provides circumstantial evidence for a physiologically vulnerable sharpening mechanism occurring within the cochlea subsequent to the displacement pattern of the basilar membrane.
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dallos P., Cheatham M. A. Travel time in the cochlea and its determination from cochlear-microphonic data. J Acoust Soc Am. 1971 Apr;49(4 Suppl):1140+–1140+. doi: 10.1121/1.1912475. [DOI] [PubMed] [Google Scholar]
- De Boer E. Reverse correlation. II. Initiation of nerve impulses in the inner ear. Proc K Ned Akad Wet C. 1969;72(2):129–151. [PubMed] [Google Scholar]
- Evans E. F. Narrow 'tuning' of cochlear nerve fibre responses in the guinea-pig. J Physiol. 1970 Feb;206(2):14P–15P. [PMC free article] [PubMed] [Google Scholar]
- Evans E. F. Narrow 'tuning' of the responses of cochlear nerve fibres emanating from the exposed basilar membrane. J Physiol. 1970 Jun;208(2):75P–76P. [PubMed] [Google Scholar]
- Evans E. F., Rosenberg J., Wilson J. P. The frequency resolving power of the cochlea. J Physiol. 1971 Jul;216(2):58P–59P. [PubMed] [Google Scholar]
- Heffner R., Heffner H., Masterton B. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. J Acoust Soc Am. 1971 Jun;49(6):1888–1895. doi: 10.1121/1.1912596. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Is resonance possible in the cochlea after all? Nature. 1969 Mar 8;221(5184):935–940. doi: 10.1038/221935a0. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Boyle A. J. Basilar membrane vibration examined with the Mössbauer technique. Science. 1967 Oct 20;158(3799):389–390. doi: 10.1126/science.158.3799.389. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Taylor K. J., Boyle A. J. Mechanics of the guinea pig colea. J Acoust Soc Am. 1970 Feb;47(2):504–509. doi: 10.1121/1.1911921. [DOI] [PubMed] [Google Scholar]
- KATSUKI Y., SUMI T., UCHIYAMA H., WATANABE T. Electric responses of auditory neurons in cat to sound stimulation. J Neurophysiol. 1958 Nov;21(6):569–588. doi: 10.1152/jn.1958.21.6.569. [DOI] [PubMed] [Google Scholar]
- KATSUKI Y., WATANABE T., SUGA N. Interaction of auditory neurons in response to two sound stimuli in cat. J Neurophysiol. 1959 Nov;22:603–623. doi: 10.1152/jn.1959.22.6.603. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y. A survey of recent developments in the study of auditory physiology. Ann Otol Rhinol Laryngol. 1968 Aug;77(4):656–675. doi: 10.1177/000348946807700406. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y., Moxon E. C., Levine R. A. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Sensorineural hearing loss. Ciba Found Symp. 1970:241–273. doi: 10.1002/9780470719756.ch15. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y., Sachs M. B., Peake W. T. Shapes of tuning curves for single auditory-nerve fibers. J Acoust Soc Am. 1967 Dec;42(6):1341–1342. doi: 10.1121/1.1910723. [DOI] [PubMed] [Google Scholar]
- Lynn P. A., Sayers B. M. Cochlear innervation, signal processing, and their relation to auditory time-intensity effects. J Acoust Soc Am. 1970 Feb;47(2):525–533. doi: 10.1121/1.1911924. [DOI] [PubMed] [Google Scholar]
- Molnar C. E., Loeffel R. G., Pfeiffer R. R. Distortion compensating, condenser-earphone driver for physiological studies. J Acoust Soc Am. 1968 May;43(5):1177–1178. doi: 10.1121/1.1910953. [DOI] [PubMed] [Google Scholar]
- NOMOTO M., SUGA N., KATSUKI Y. DISCHARGE PATTERN AND INHIBITION OF PRIMARY AUDITORY NERVE FIBERS IN THE MONKEY. J Neurophysiol. 1964 Sep;27:768–787. doi: 10.1152/jn.1964.27.5.768. [DOI] [PubMed] [Google Scholar]
- Nieder P. Addressed exponential delay line theory of cochlear organization. Nature. 1971 Mar 26;230(5291):255–257. doi: 10.1038/230255a0. [DOI] [PubMed] [Google Scholar]
- Rhode W. S. Observations of the vibration of the basilar membrane in squirrel monkeys using the Mössbauer technique. J Acoust Soc Am. 1971 Apr;49(4 Suppl):1218+–1218+. doi: 10.1121/1.1912485. [DOI] [PubMed] [Google Scholar]
- Sachs M. B., Kiang N. Y. Two-tone inhibition in auditory-nerve fibers. J Acoust Soc Am. 1968 May;43(5):1120–1128. doi: 10.1121/1.1910947. [DOI] [PubMed] [Google Scholar]
- Scharf B., Hellman R. P. Model of loudness summation applied to impaired ears. J Acoust Soc Am. 1966 Jul;40(1):71–78. doi: 10.1121/1.1910066. [DOI] [PubMed] [Google Scholar]
- Simmons F. B., Linehan J. A. Observations on a single auditory nerve fiber over a six-week period. J Neurophysiol. 1968 Nov;31(6):799–805. doi: 10.1152/jn.1968.31.6.799. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Degeneration behaviour of the cochlear nerve. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1971;200(4):275–291. doi: 10.1007/BF00373310. [DOI] [PubMed] [Google Scholar]
- TASAKI I. Nerve impulses in individual auditory nerve fibers of guinea pig. J Neurophysiol. 1954 Mar;17(2):97–122. doi: 10.1152/jn.1954.17.2.97. [DOI] [PubMed] [Google Scholar]
- von Békésy G. Travelling waves as frequency analysers in the cochlea. Nature. 1970 Mar 28;225(5239):1207–1209. doi: 10.1038/2251207a0. [DOI] [PubMed] [Google Scholar]


