Abstract
1. The cerebellar integration of sensory inputs to Deiters neurones was investigated in decerebrate cats. In some preparations decerebration was combined with transection of the olivocerebellar fibres.
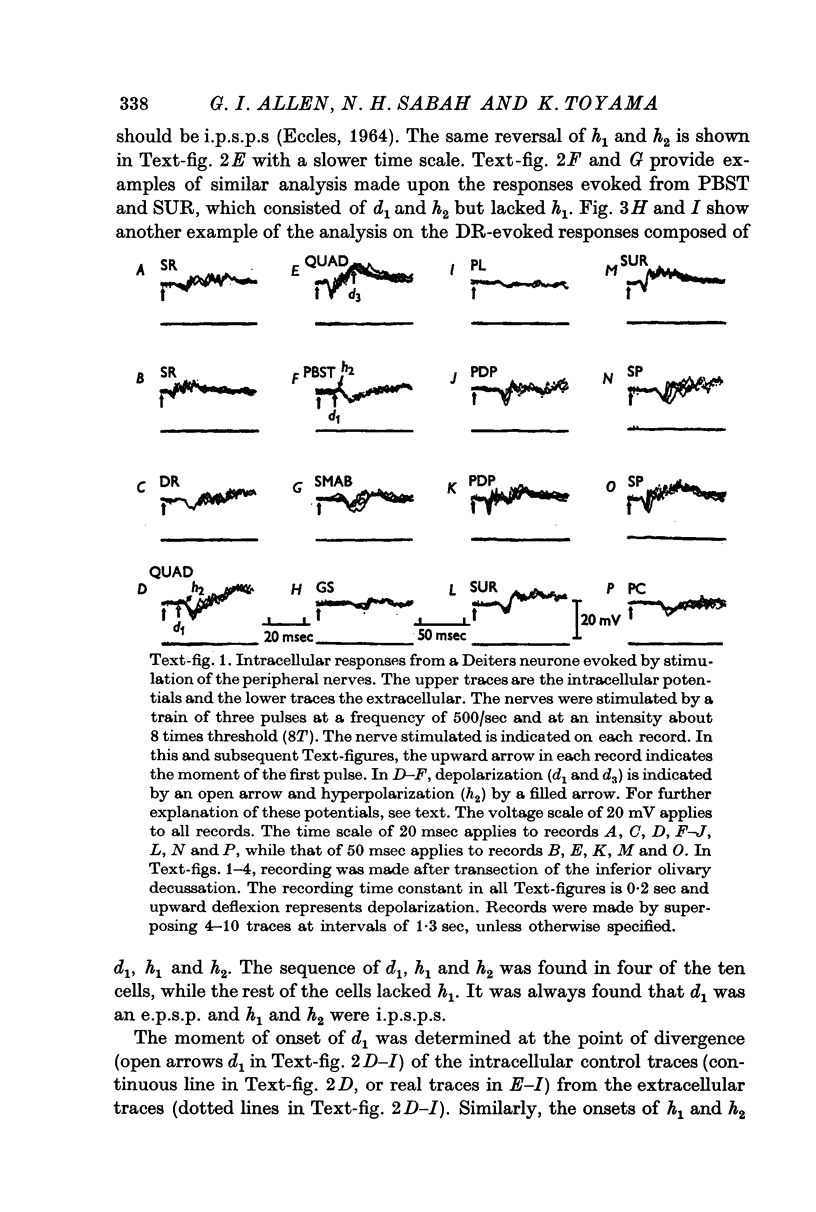
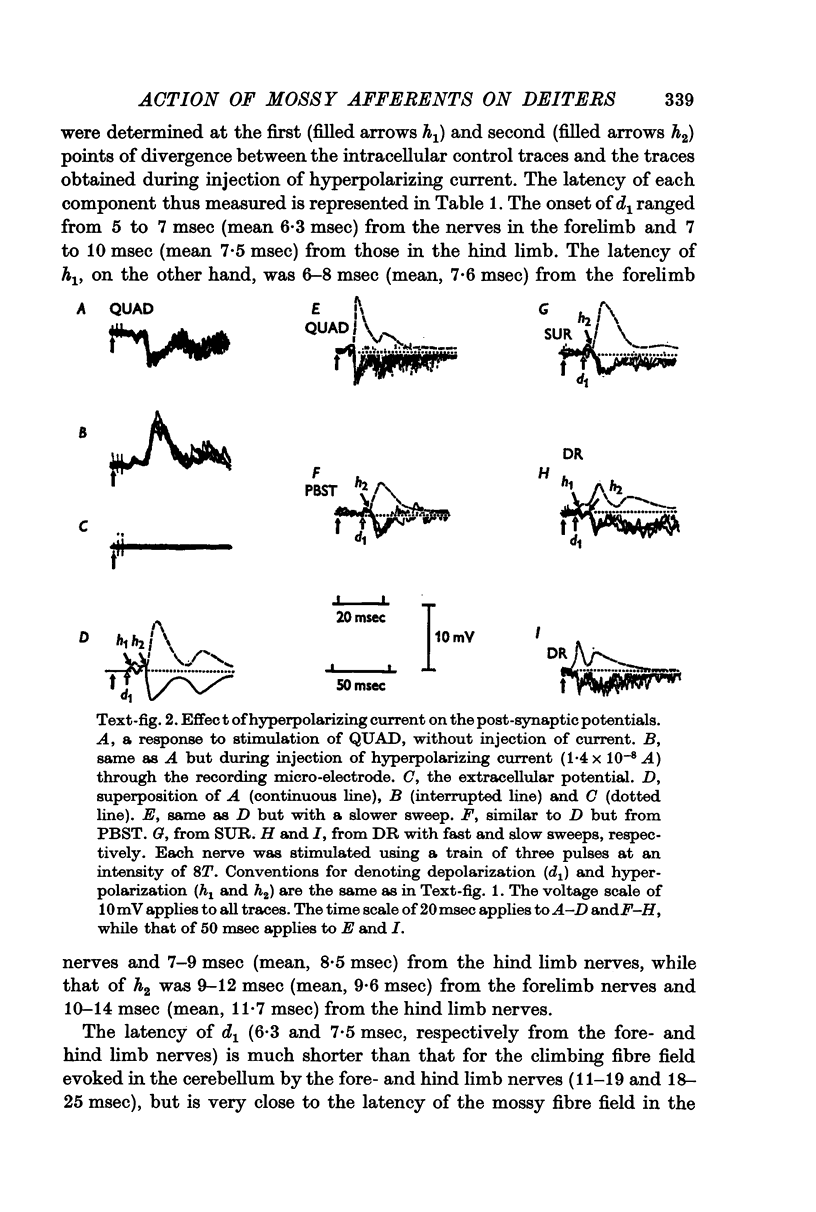
2. In the latter preparations peripheral nerve impulses generally produced a response consisting of a sequence of the following post-synaptic potentials: (i) an initial e.p.s.p. (d1), (ii) early i.p.s.p. (h1), (iii) later i.p.s.p. (h2).
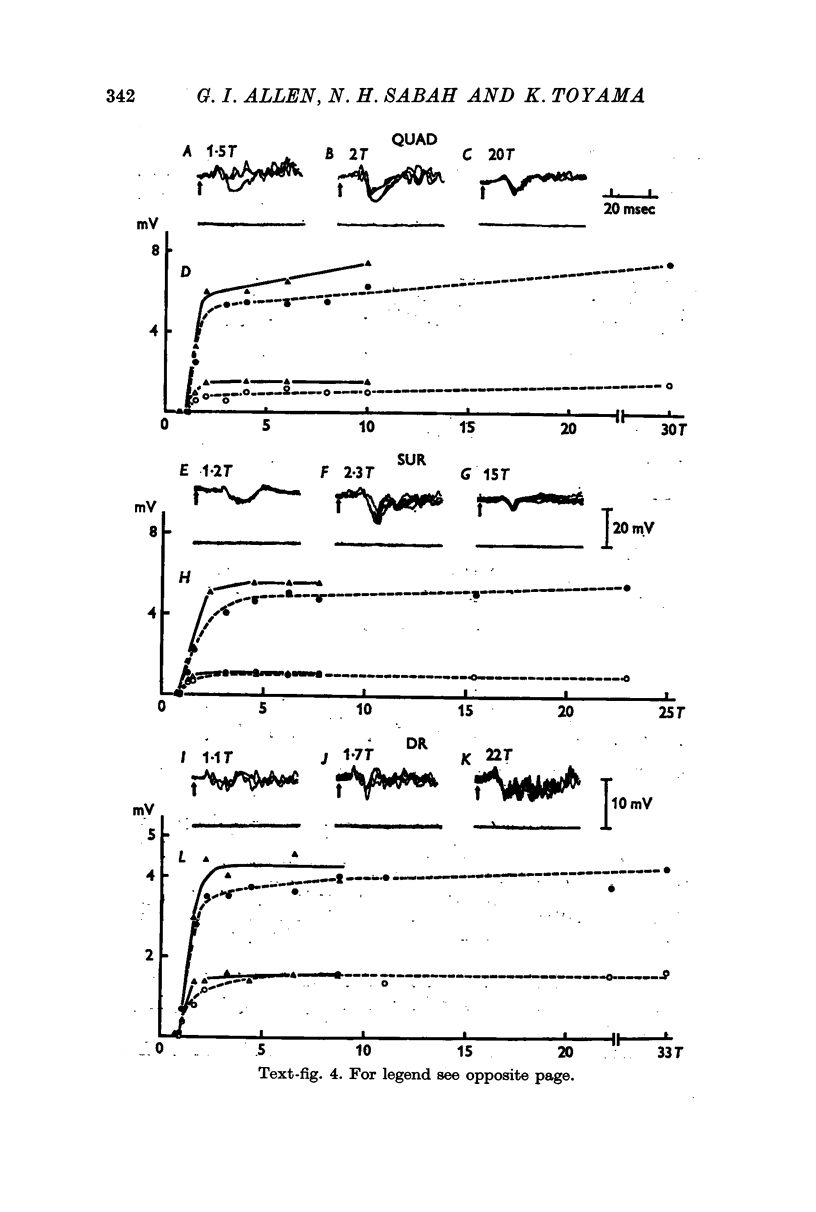
3. The mean latencies of d1, h1 and h2 were 5·7, 7·3 and 9·8 msec from the forelimb nerves, and 7·5, 9·0 and 13·4 msec from the hind limb nerves, respectively.
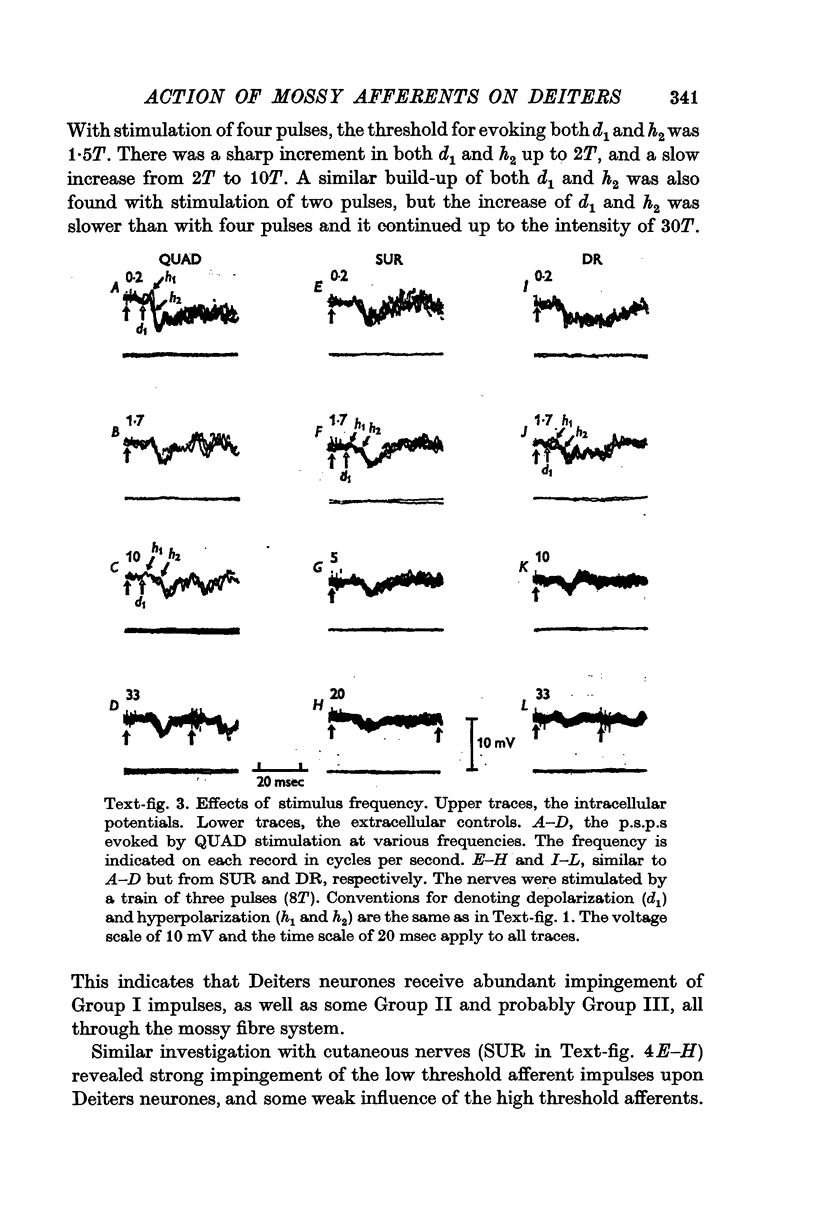
4. The stimulus intensity—response relation indicates that the Group I muscle afferents as well as the low threshold cutaneous afferents contribute to the response.
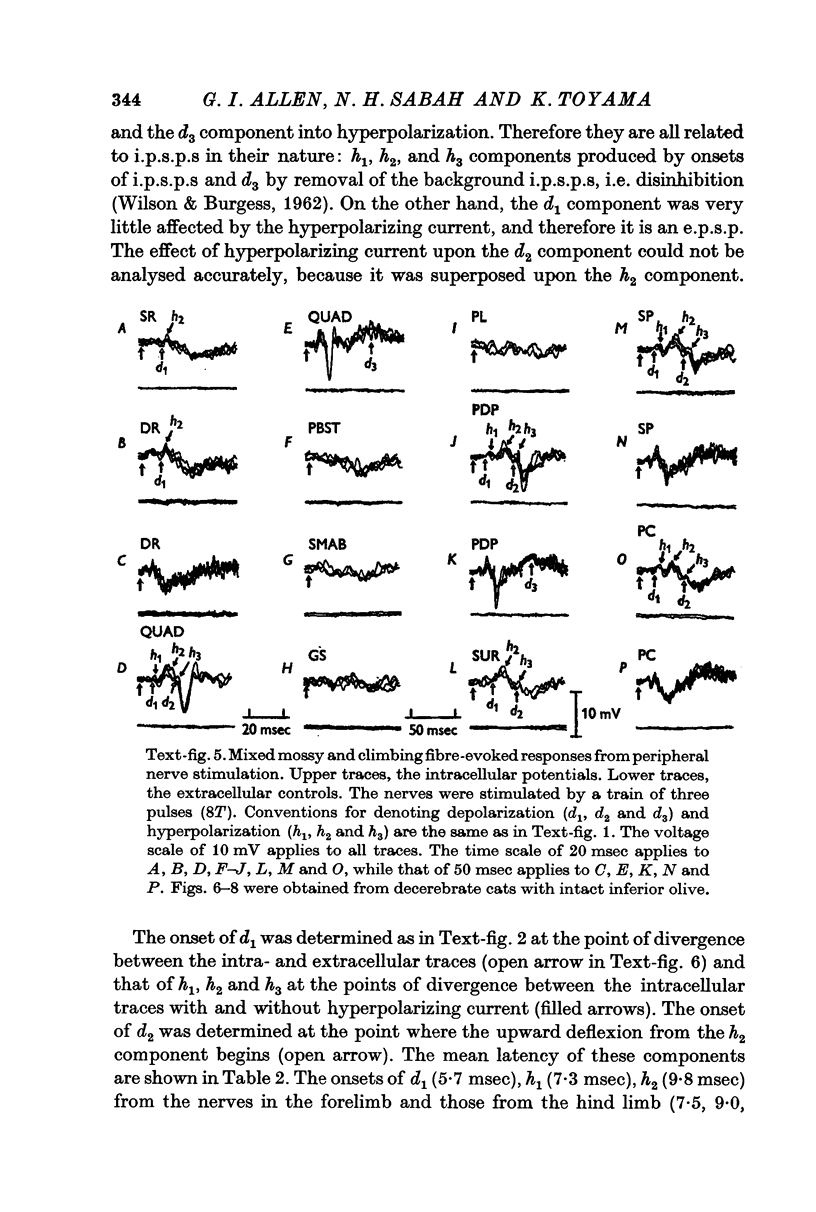
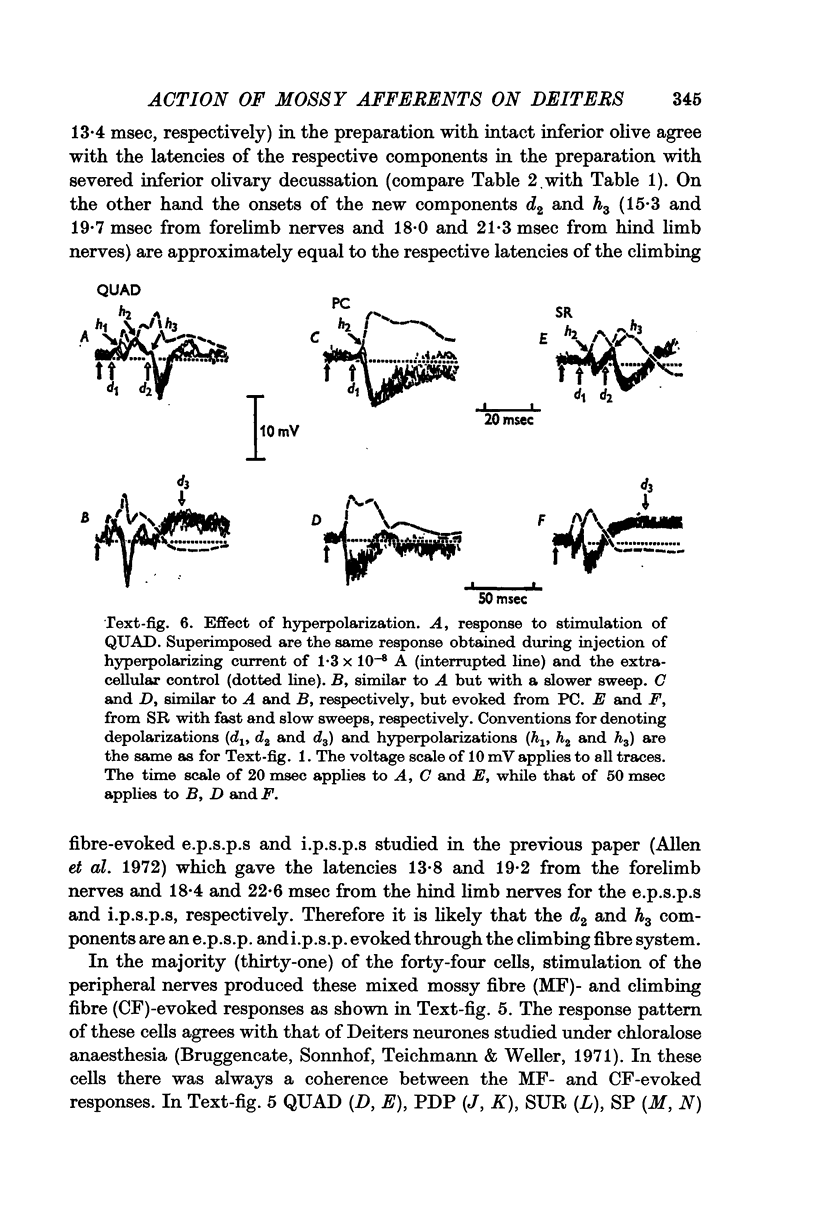
5. In the preparations with the intact inferior olive there were additional components of the post-synaptic potentials: a later e.p.s.p. (d2) and another later i.p.s.p. (h3), their mean latencies being 15·3 and 19·7 msec from the forelimb nerves, and 18·0 and 21·3 msec from the hind limb nerves, respectively.
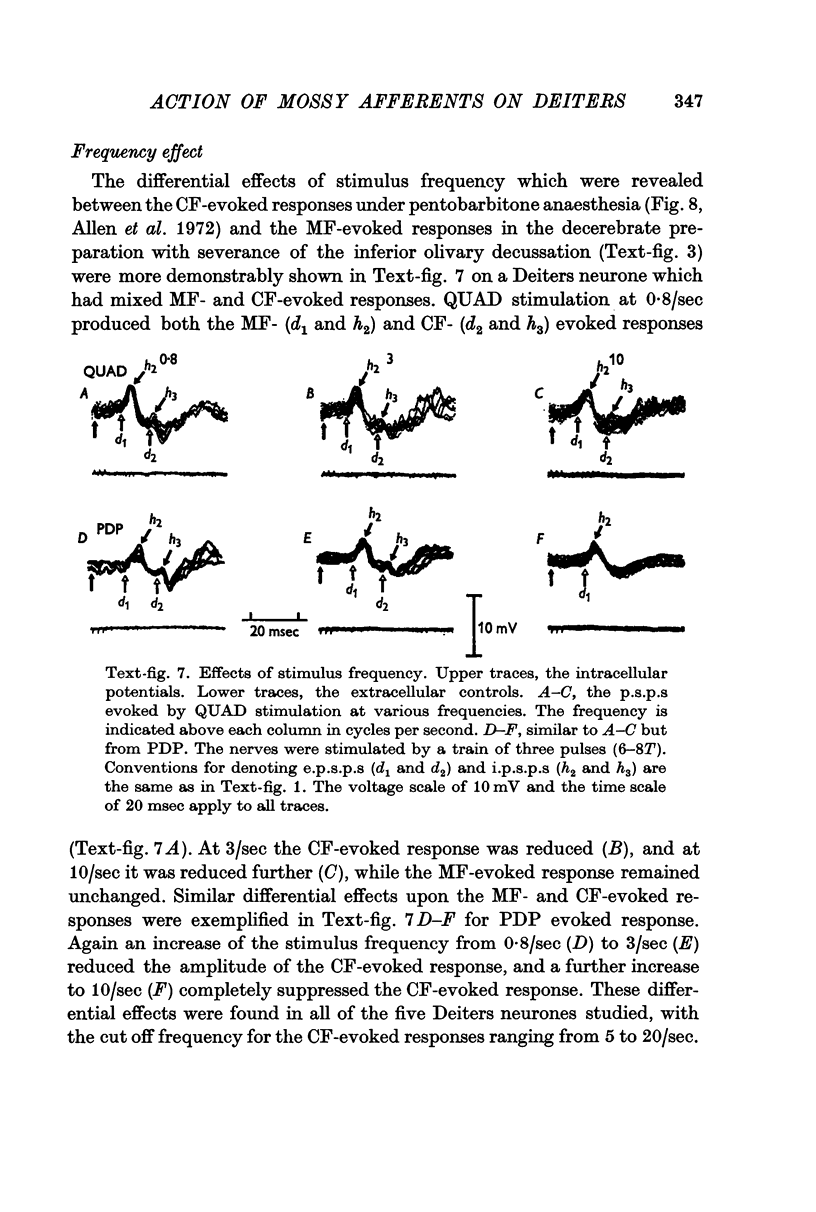
6. The d1 and h2 components were attributed to the mossy fibre afferents and d2 and h3 to the climbing fibres; d1 and d2 were due to excitation through the collaterals of the mossy and climbing fibres, and h2 and h3 to inhibition from Purkyně cells activated by the mossy and climbing fibres, respectively. h1 was too early to be produced through the cerebellum, and was probably mediated by inhibitory neurones in the reticular formation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Sabah N. H., Toyama K. Effect of fore- and hindlimb nerve stimulation on Deiters' neurones. Brain Res. 1971 Feb 5;25(3):645–650. doi: 10.1016/0006-8993(71)90469-0. [DOI] [PubMed] [Google Scholar]
- Allen G. I., Sabah N. H., Toyama K. Synaptic actions of peripheral nerve impulses upon Deiters neurones via the climbing fibre afferents. J Physiol. 1972 Oct;226(2):311–333. doi: 10.1113/jphysiol.1972.sp009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batini C., Pumain R. Activation of Purkinje neurons through climbing fibres after chronic lesions of the olivo-cerebellar pathway. Experientia. 1968 Sep 15;24(9):914–916. doi: 10.1007/BF02138651. [DOI] [PubMed] [Google Scholar]
- Bruggencate G. T., Sonnhof U., Teichmann R., Weller E. A study of the synaptic input to Deiters' neurones evoked by stimulation of peripheral nerves and spinal cord. Brain Res. 1971 Jan 8;25(1):207–211. doi: 10.1016/0006-8993(71)90584-1. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Afferent volleys in limb nerves influencing impulse discharges in cerebellar cortex. II. In Purkyne cells. Exp Brain Res. 1971 Jul 26;13(1):36–53. [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Firing patterns of Purkinje cells in response to volleys from limb nerves. Brain Res. 1969 Jun;14(1):222–226. doi: 10.1016/0006-8993(69)90043-2. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Investigations on integration of mossy fiber inputs to Purkynè cells in the anterior lobe. Exp Brain Res. 1971 Jul 26;13(1):54–77. [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Analysis of electrical potentials evoked in the cerebellar anterior lobe by stimulation of hindlimb and forelimb nerves. Exp Brain Res. 1968;6(3):171–194. doi: 10.1007/BF00235123. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Topographical investigations on the climbing fiber inputs from forelimb and hindlimb afferents to the cerebellar anterior lobe. Exp Brain Res. 1968;6(3):195–215. doi: 10.1007/BF00235124. [DOI] [PubMed] [Google Scholar]
- ITO M., HONGO T., YOSHIDA M., OKADA Y., OBATA K. ANTIDROMIC AND TRANS-SYNAPTIC ACTIVATION OF DEITERS' NEURONES INDUCED FROM THE SPINAL CORD. Jpn J Physiol. 1964 Dec 15;14:638–658. doi: 10.2170/jjphysiol.14.638. [DOI] [PubMed] [Google Scholar]
- Ito M., Udo M., Mano N. Long inhibitory and excitatory pathways converging onto cat reticular and Deiters' neurons and their relevance to reticulofugal axons. J Neurophysiol. 1970 Mar;33(2):210–226. doi: 10.1152/jn.1970.33.2.210. [DOI] [PubMed] [Google Scholar]
- Koller E. A., Jenny M. A technique for standard decerebration by high-frequency coagulation. Brain Res. 1969 Jul;14(2):549–552. doi: 10.1016/0006-8993(69)90137-1. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary J. L., Dunsker S. B., Smith J. M., Inukai J., O'Leary M. Termination of the olivocerebellar system in the cat. Arch Neurol. 1970 Mar;22(3):193–206. doi: 10.1001/archneur.1970.00480210003001. [DOI] [PubMed] [Google Scholar]
- Udo M., Mano N. Discrimination of different spinal monosynaptic pathways converging into reticular neurons. J Neurophysiol. 1970 Mar;33(2):227–238. doi: 10.1152/jn.1970.33.2.227. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., BURGESS P. R. Disinhibition in the cat spinal cord. J Neurophysiol. 1962 May;25:392–404. doi: 10.1152/jn.1962.25.3.392. [DOI] [PubMed] [Google Scholar]