Abstract
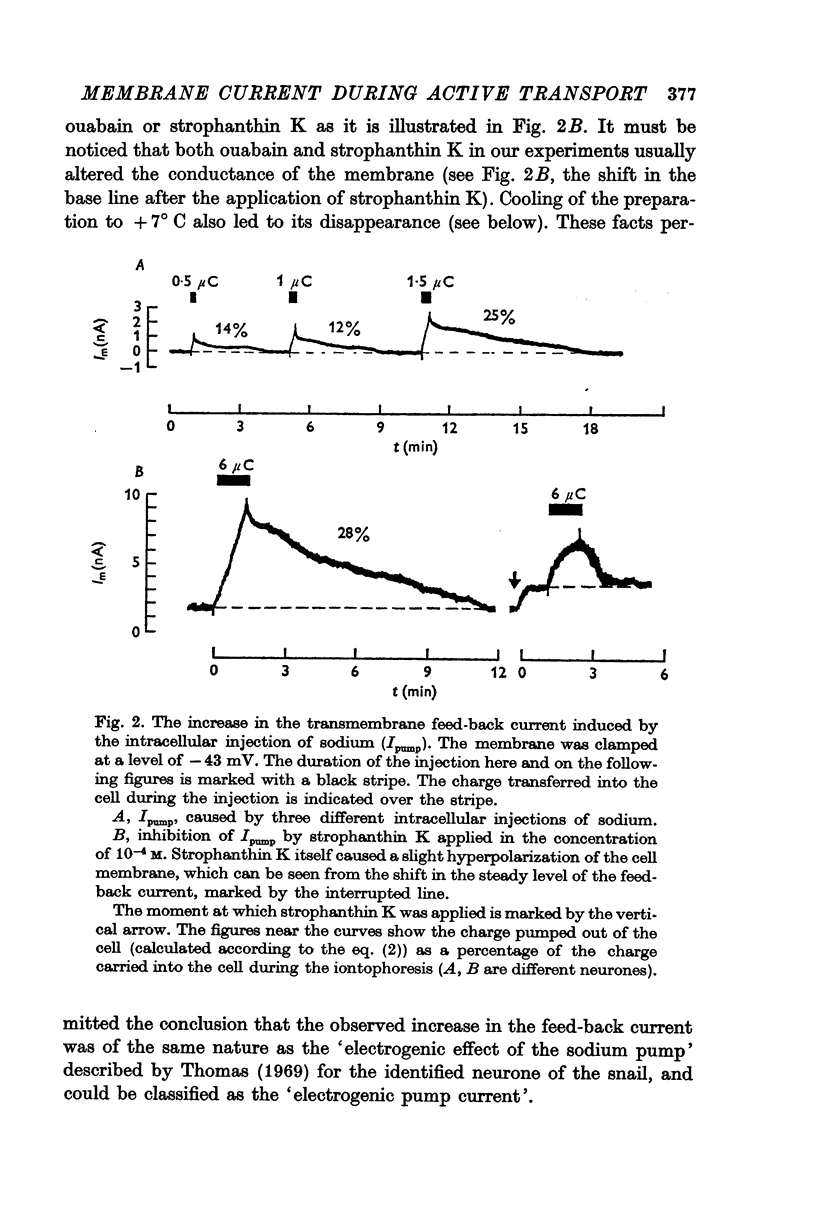
1. The membrane current caused by the iontophoretic injection of sodium into giant neurones of the snail Helix pomatia was investigated under a long lasting voltage clamp. The inhibition of this current by ouabain (10-4 M) and by cooling to + 7° C confirmed its link with the active transport of ions. Therefore this current is called the pump current.
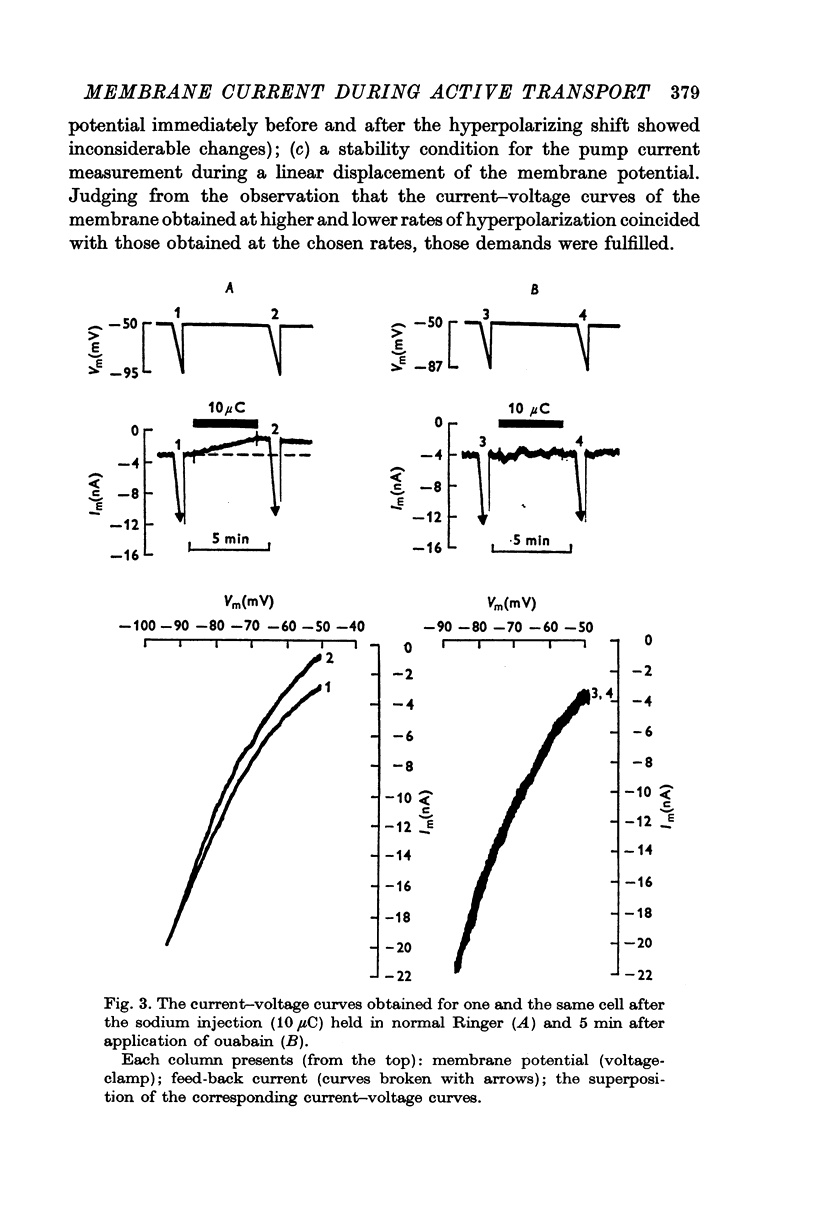
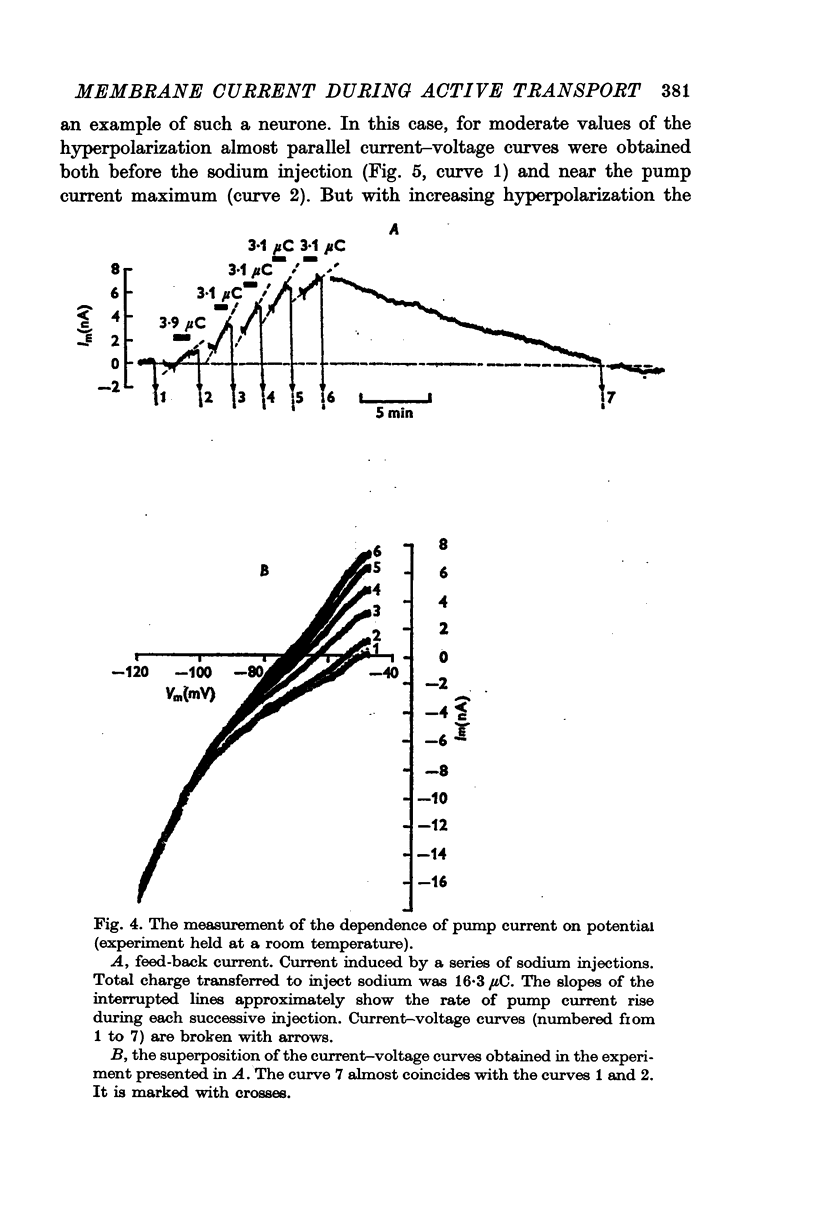
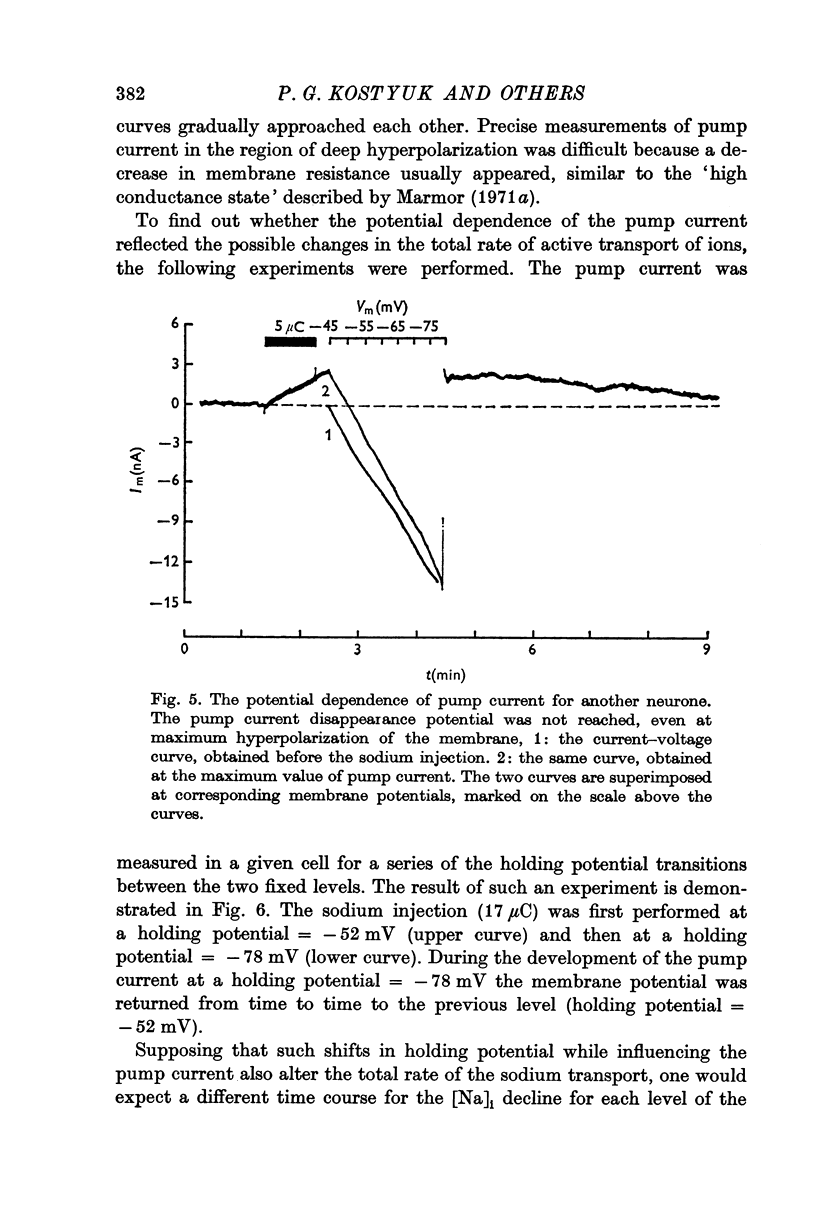
2. Over the range of membrane potential -40 to -100 mV the changes in the steady current—voltage curves caused by the pump current development were investigated. The pump current was found to be potential-dependent. It decreased with increasing hyperpolarization of the neurone.
3. With large hyperpolarizations the current—voltage curves obtained before the sodium injection and after eliciting the pump current coincided with each other. An increase in the membrane conductance was observed over the range of membrane potential corresponding to the pump current display.
4. The applied sodium injections did not cause any marked changes in the passive permeability of the membrane. This fact made it possible to measure the charge transferred across the membrane during operation of the pump current. Unlike previous data, the ratio of this value to the charge used to inject sodium into the neurone appeared to be a variable.
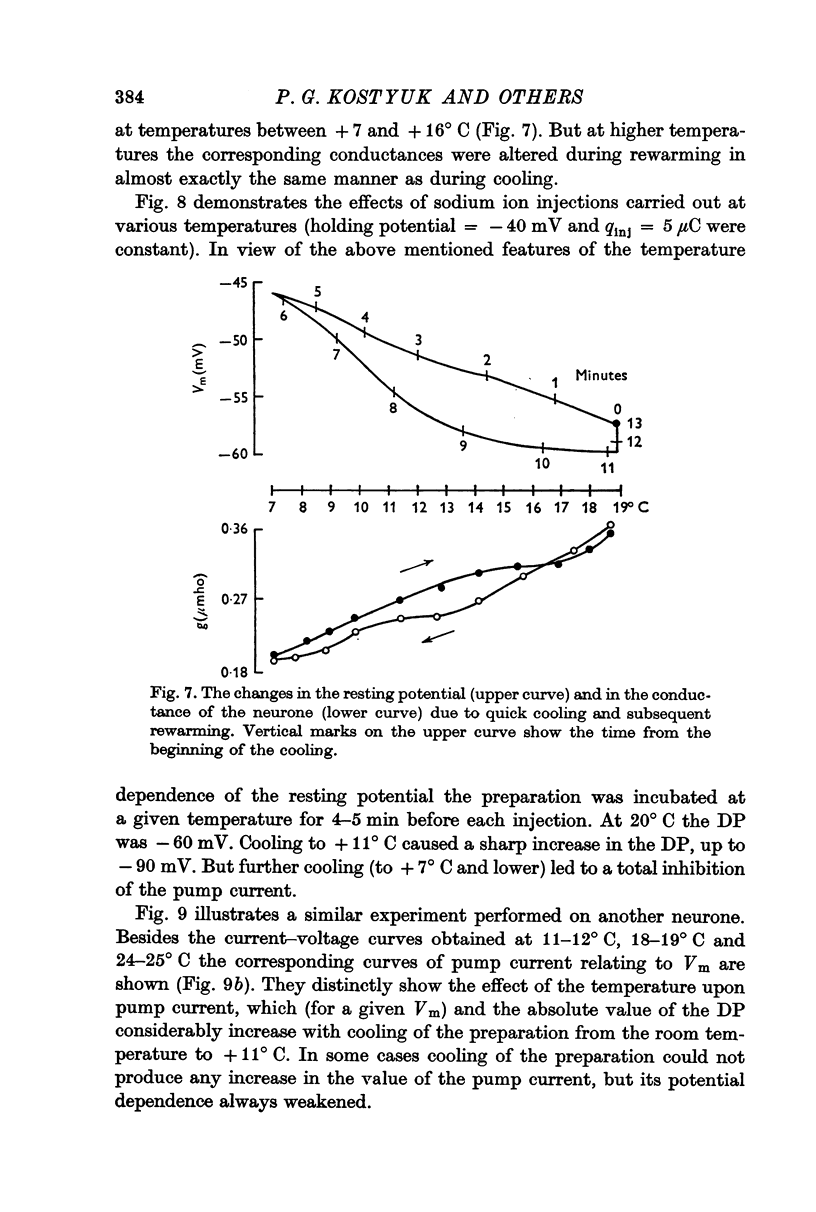
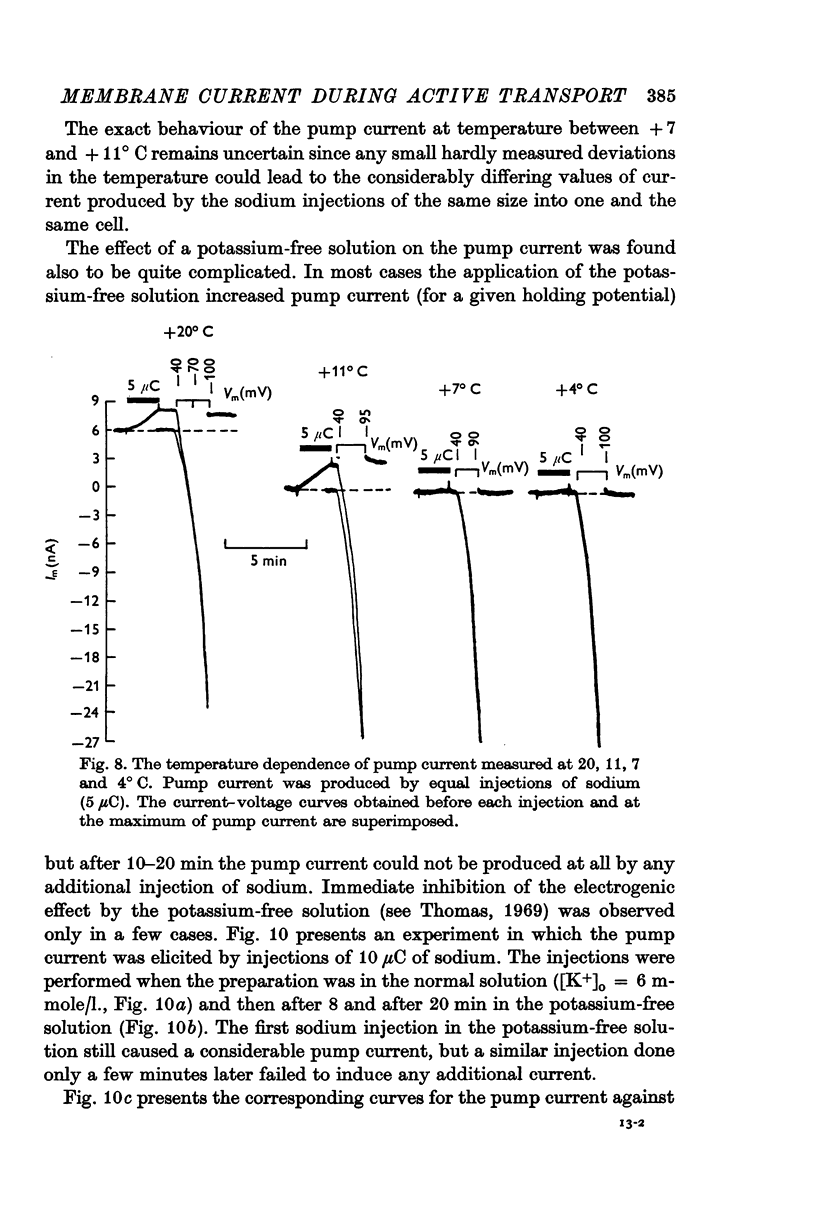
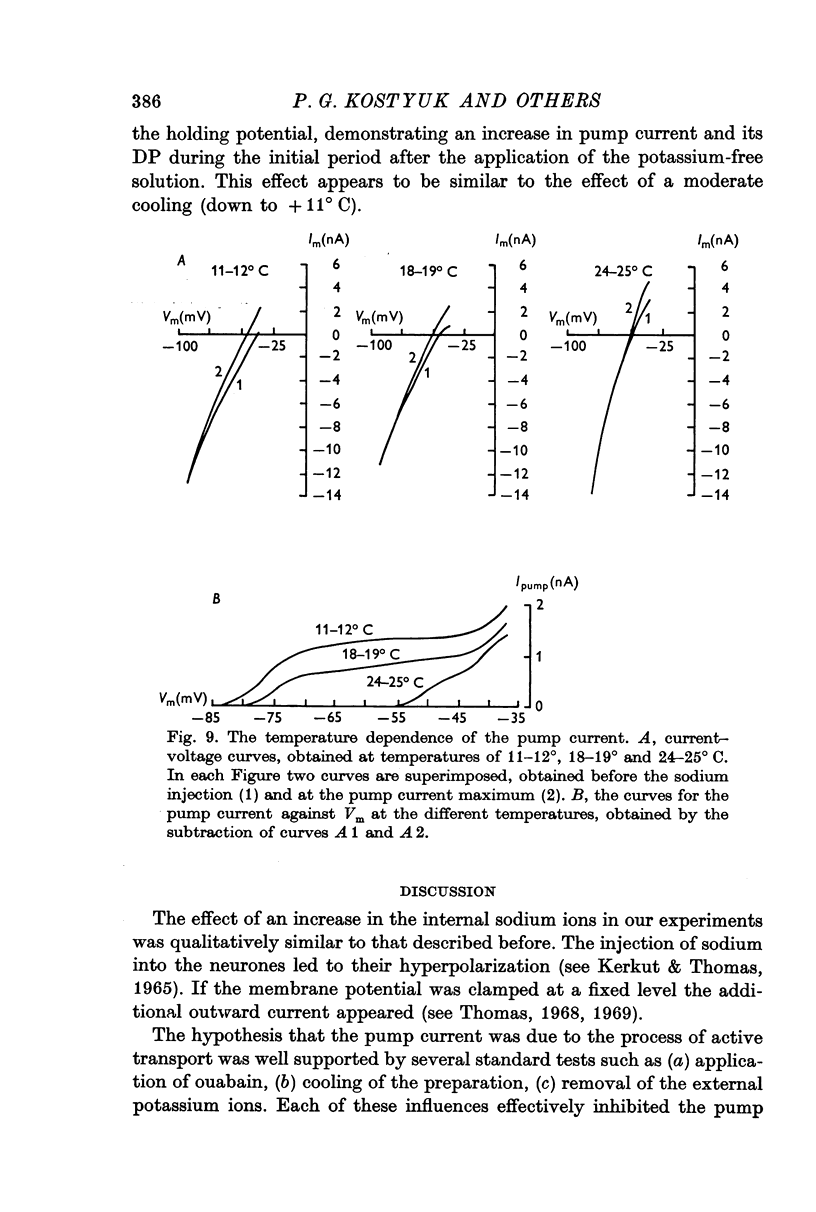
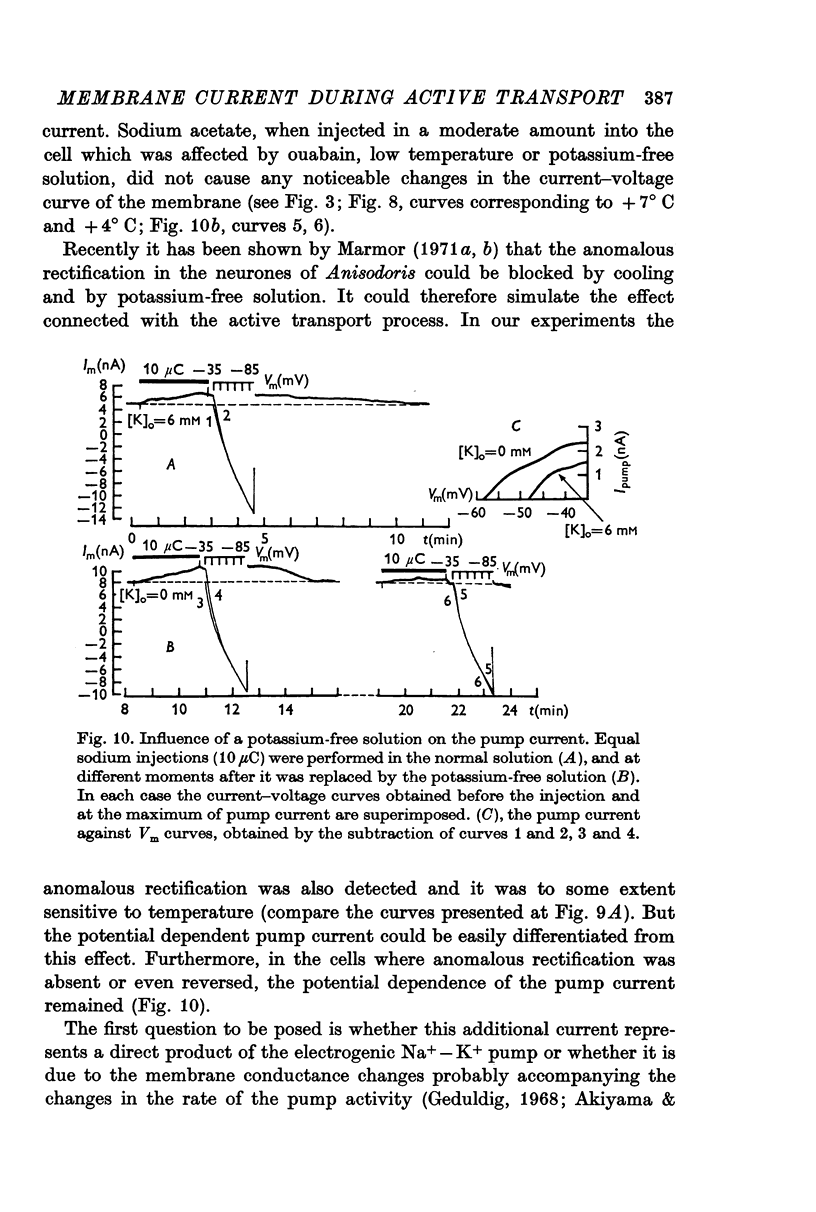
5. When the preparation was cooled to + 11° C, and also during the first few minutes after the application of a potassium-free solution, both the pump current and the membrane potential at which it disappeared could increase.
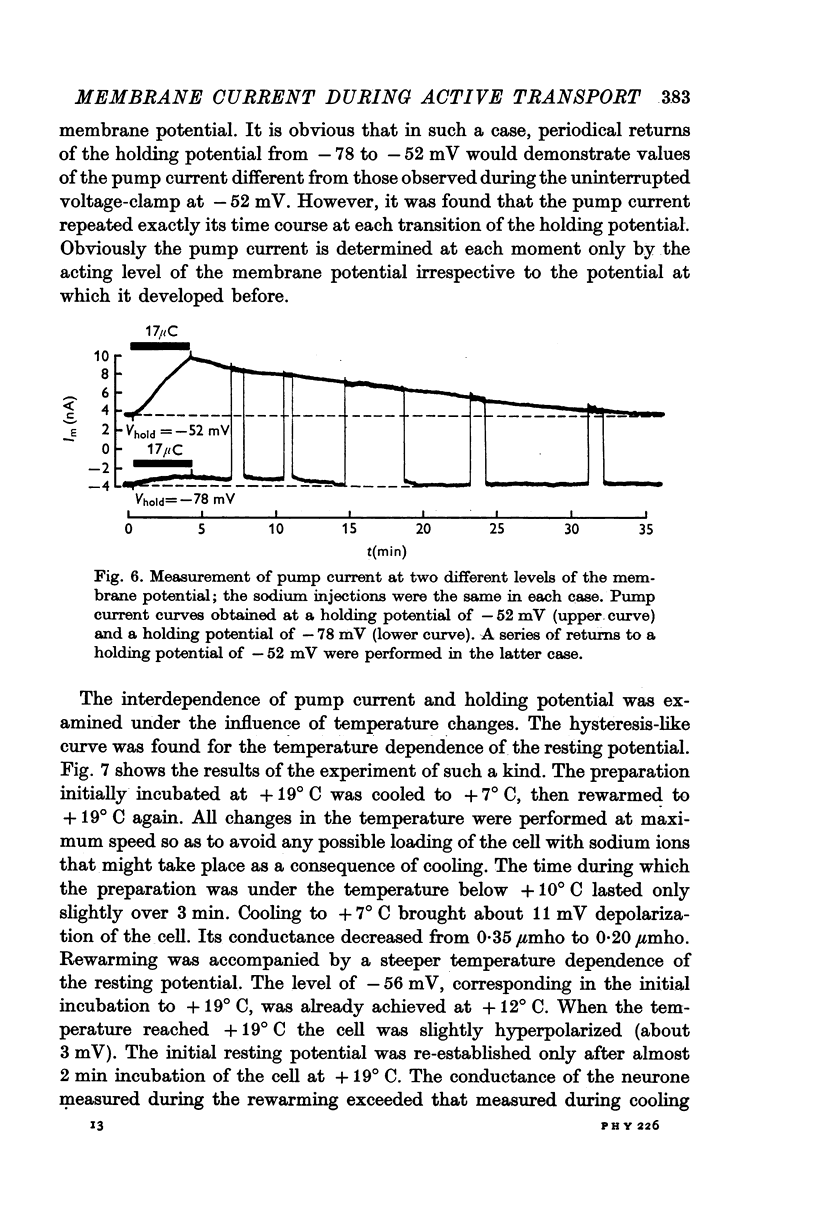
6. The pump current measurements during a number of transitions from one fixed level of the membrane potential to another showed that the current did not depend upon the potential at which it developed before each transition.
7. The data presented allow the suggestion that the potential dependence of the pump current is determined by the changes in the rate of active transport of potassium, while the rate of active transport of sodium remains constant.
Full text
PDF

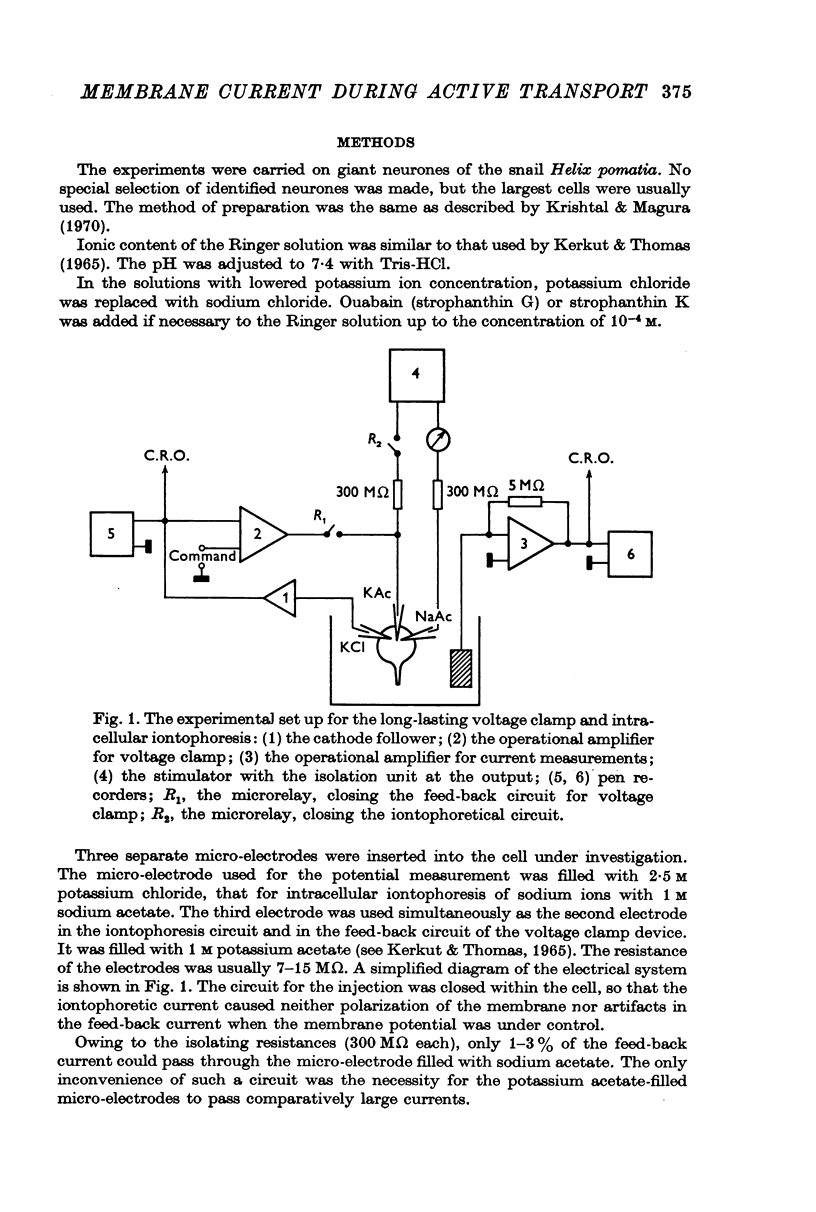








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Grundfest H. The hyperpolarization of frog skeletal muscle fibres induced by removing potassium from the bathing medium. J Physiol. 1971 Aug;217(1):33–60. doi: 10.1113/jphysiol.1971.sp009558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P. Effects of intracellular adenosine-5'-diphosphate and orthophosphate on the sensitivity of sodium efflux from squid axon to external sodium and potassium. J Gen Physiol. 1970 Nov;56(5):583–620. doi: 10.1085/jgp.56.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D. A ouabain-sensitive membrane conductance. J Physiol. 1968 Feb;194(2):521–533. doi: 10.1113/jphysiol.1968.sp008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov V. D., Kostiuk P. G., Maiskii V. A. Izmeneniia élektricheskikh kharakteristik membrany gigantskogo neirona pri uvelichenii naruzhnoi kontsentratsii ionov kaliia. Biofizika. 1965;10(2):272–280. [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Magura I. S. Calcium ions as inward current carriers in mollusc neurones. Comp Biochem Physiol. 1970 Aug 15;35(4):857–866. doi: 10.1016/0010-406x(70)90080-0. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., AWAD M. Z. THE CONTROL OF THE MEMBRANE POTENTIAL OF MUSCLE FIBERS BY THE SODIUM PUMP. J Gen Physiol. 1965 May;48:761–775. doi: 10.1085/jgp.48.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. F. The effects of temperature and ions on the current-voltage relation and electrical characteristics of a molluscan neurone. J Physiol. 1971 Nov;218(3):573–598. doi: 10.1113/jphysiol.1971.sp009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. F. The independence of electrogenic sodium transport and membrane potential in a molluscan neurone. J Physiol. 1971 Nov;218(3):599–608. doi: 10.1113/jphysiol.1971.sp009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton R. B. An investigation of the electrogenic sodium pump in snail neurones, using the constant-field theory. J Exp Biol. 1969 Aug;51(1):181–201. doi: 10.1242/jeb.51.1.181. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Measurement of current produced by the sodium pump in a snail neurone. J Physiol. 1968 Mar;195(2):23P–24P. [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]


