Abstract
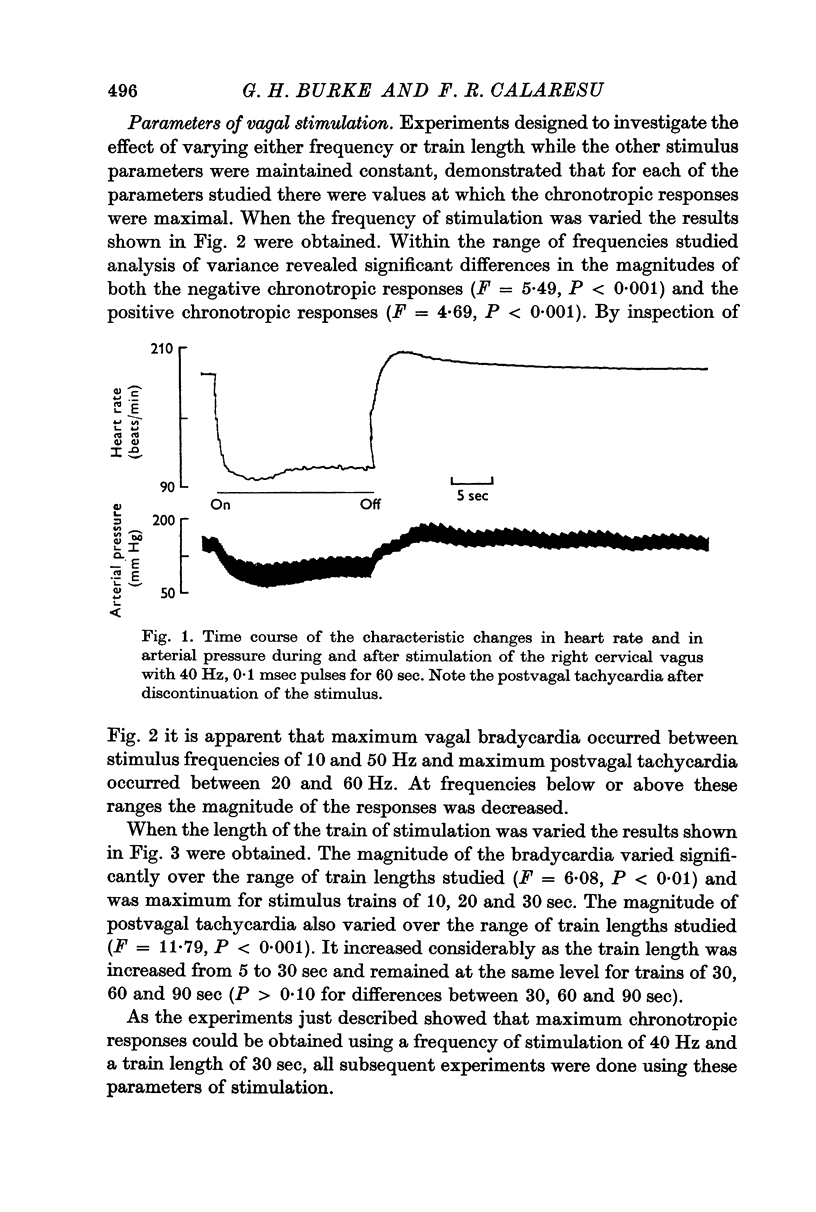
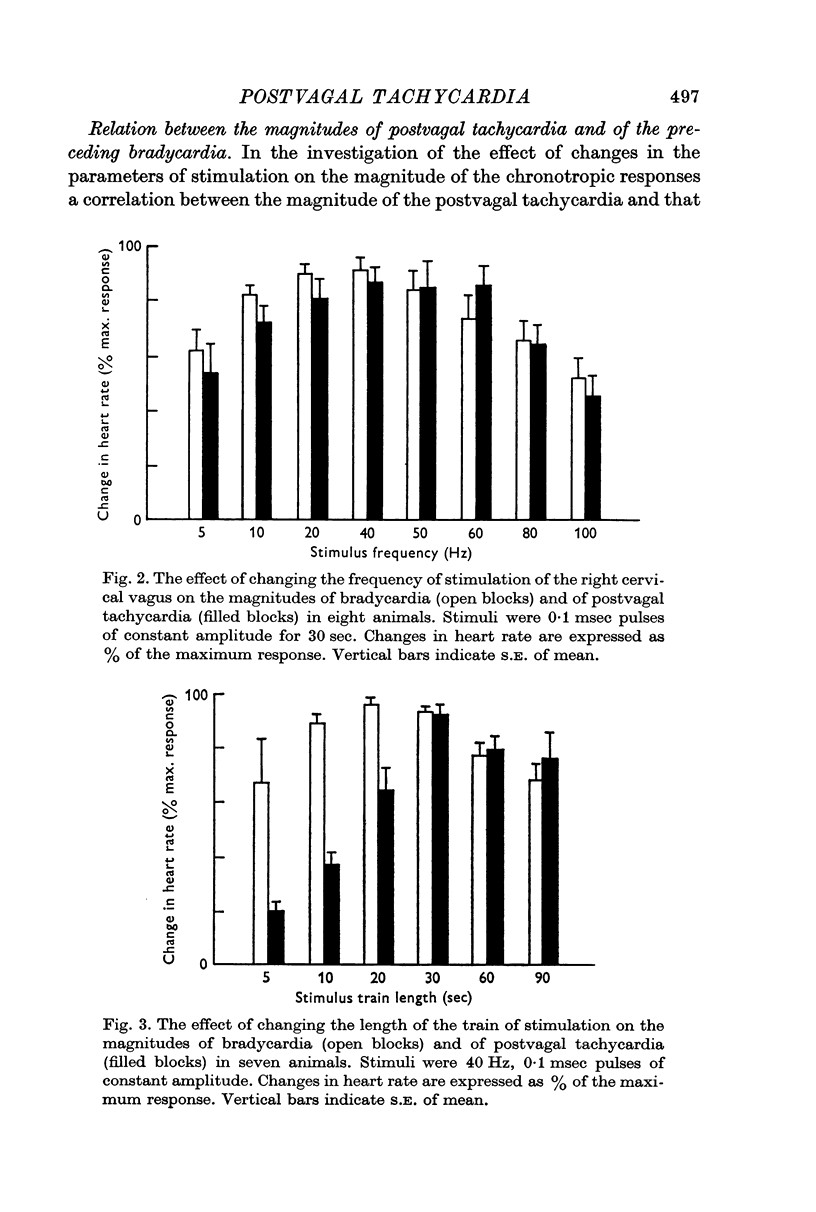
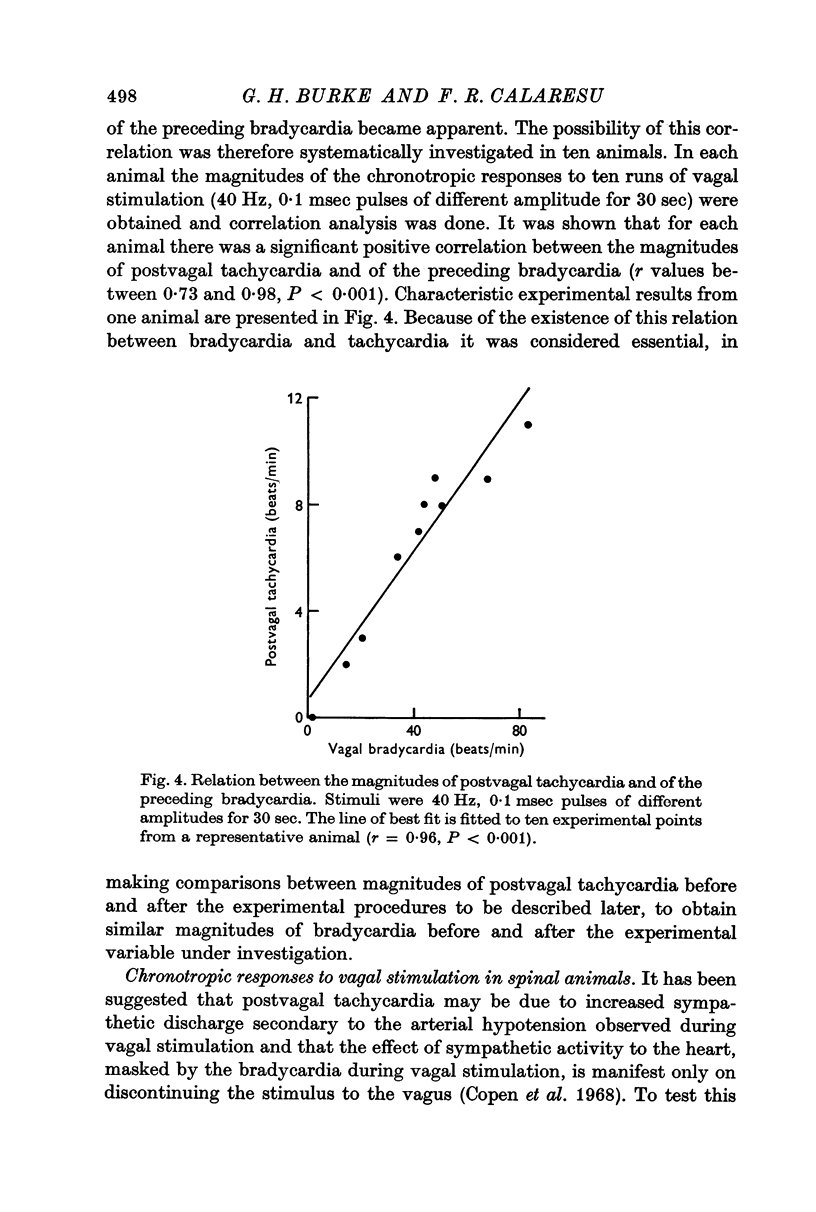
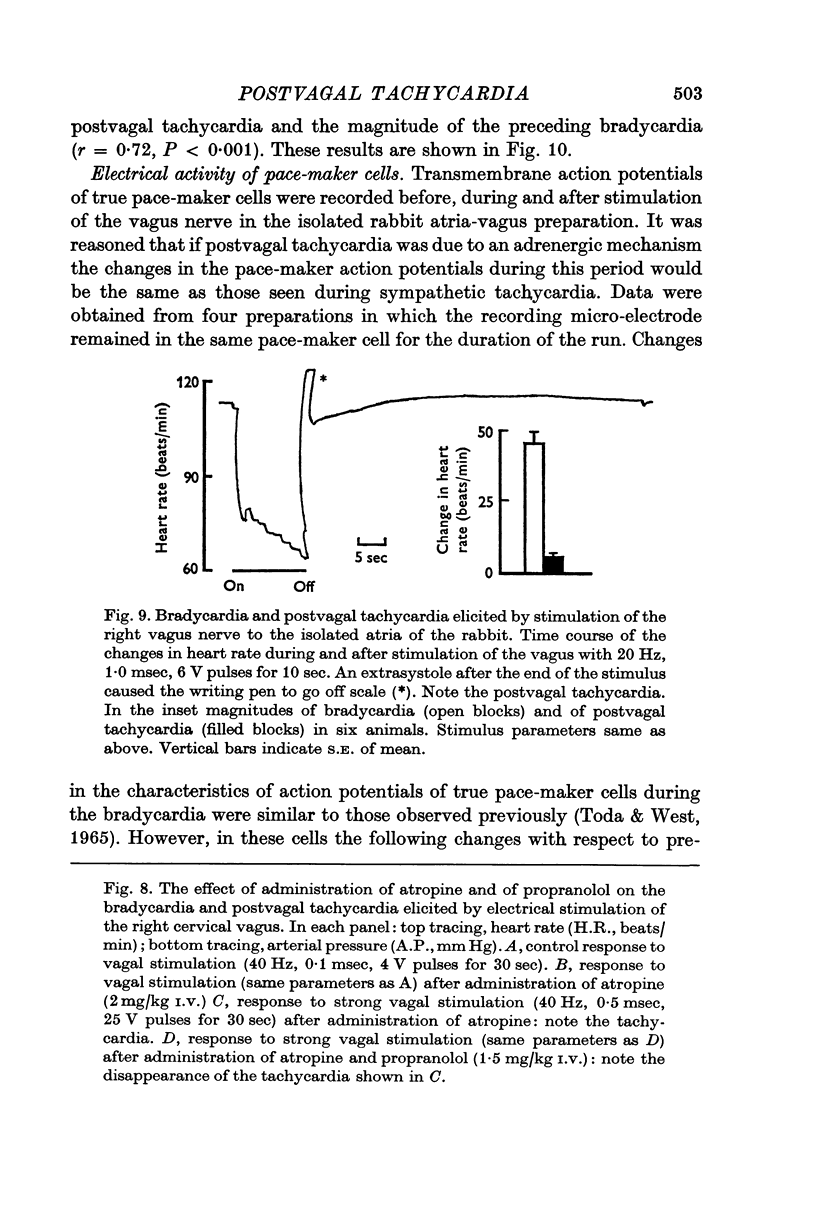
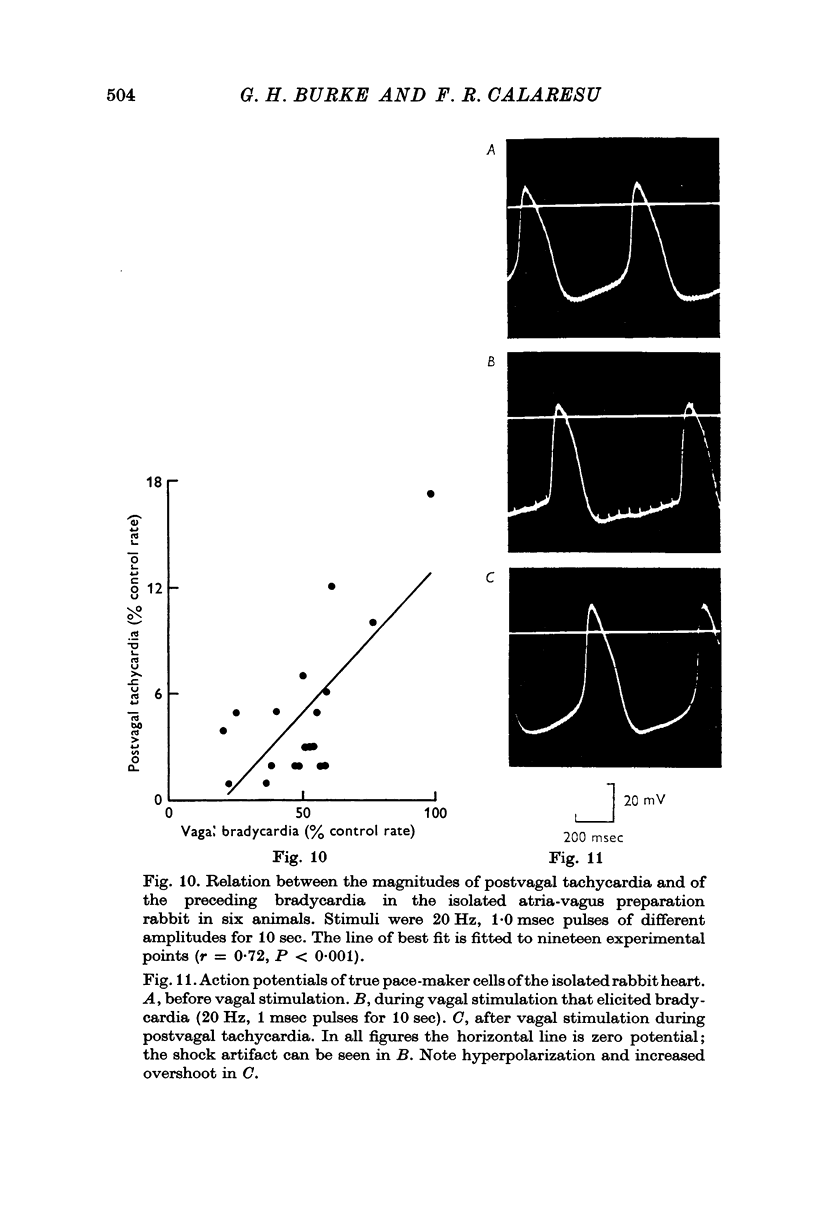
1. Postvagal tachycardia, the transient increase in heart rate that follows the sinus bradycardia elicited by vagal stimulation, was investigated in thirty chloralosed cats. Maximum postvagal tachycardia was elicited by stimulation at frequencies of 20-60 Hz with train durations of 30-90 sec. A positive correlation was demonstrated between the magnitudes of postvagal tachycardia and of the preceding sinus bradycardia.
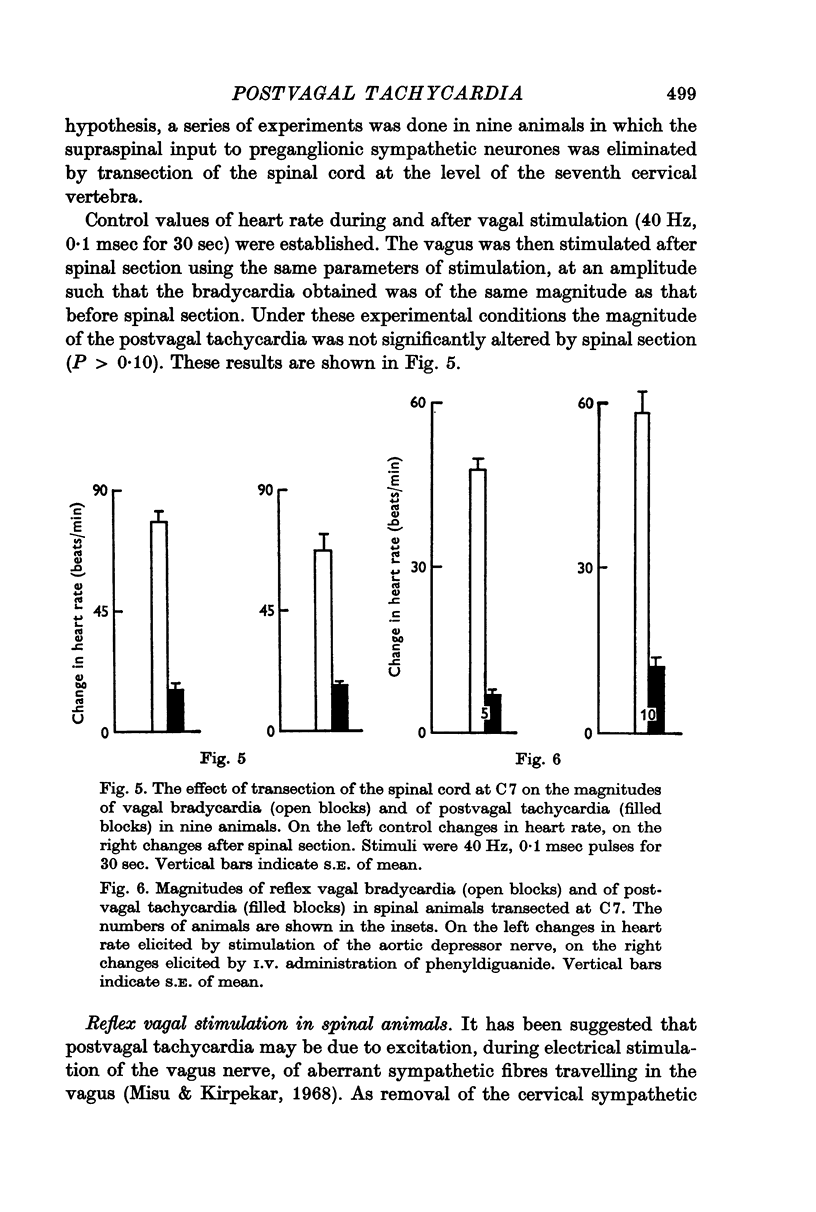
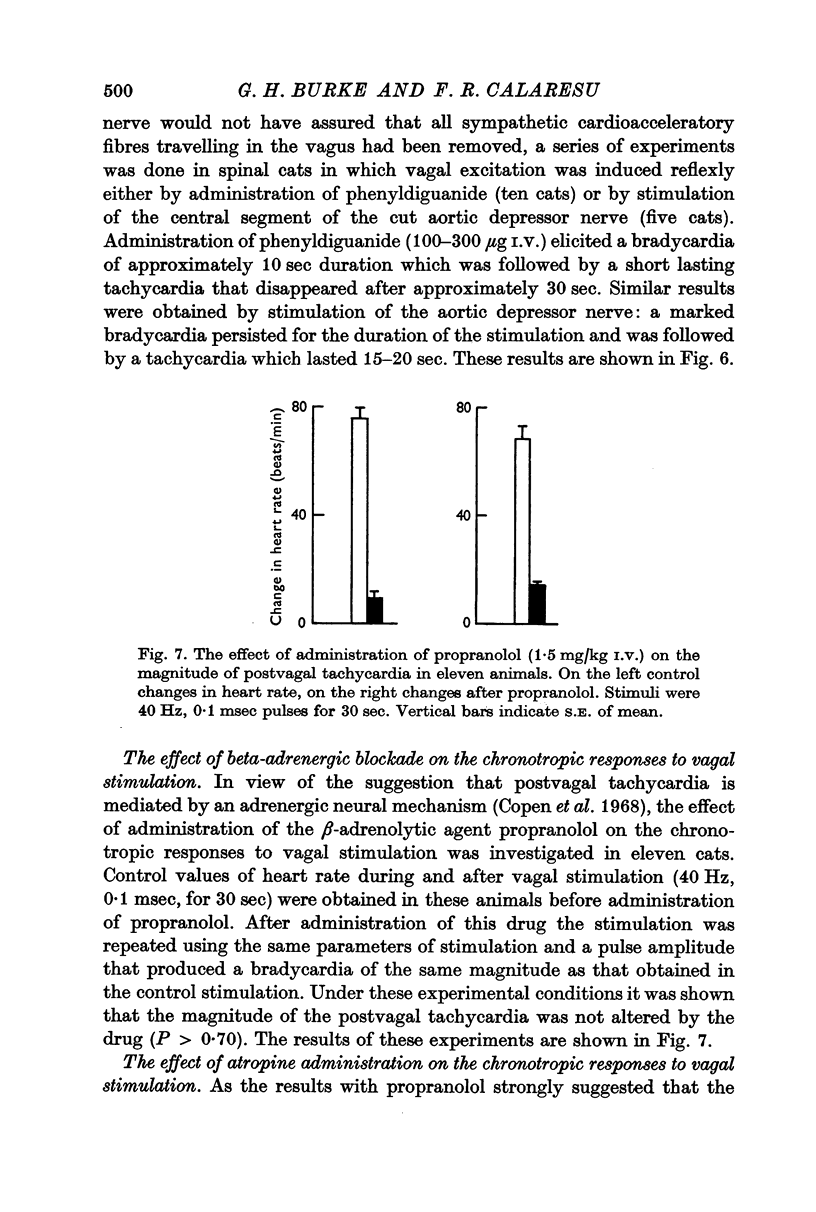
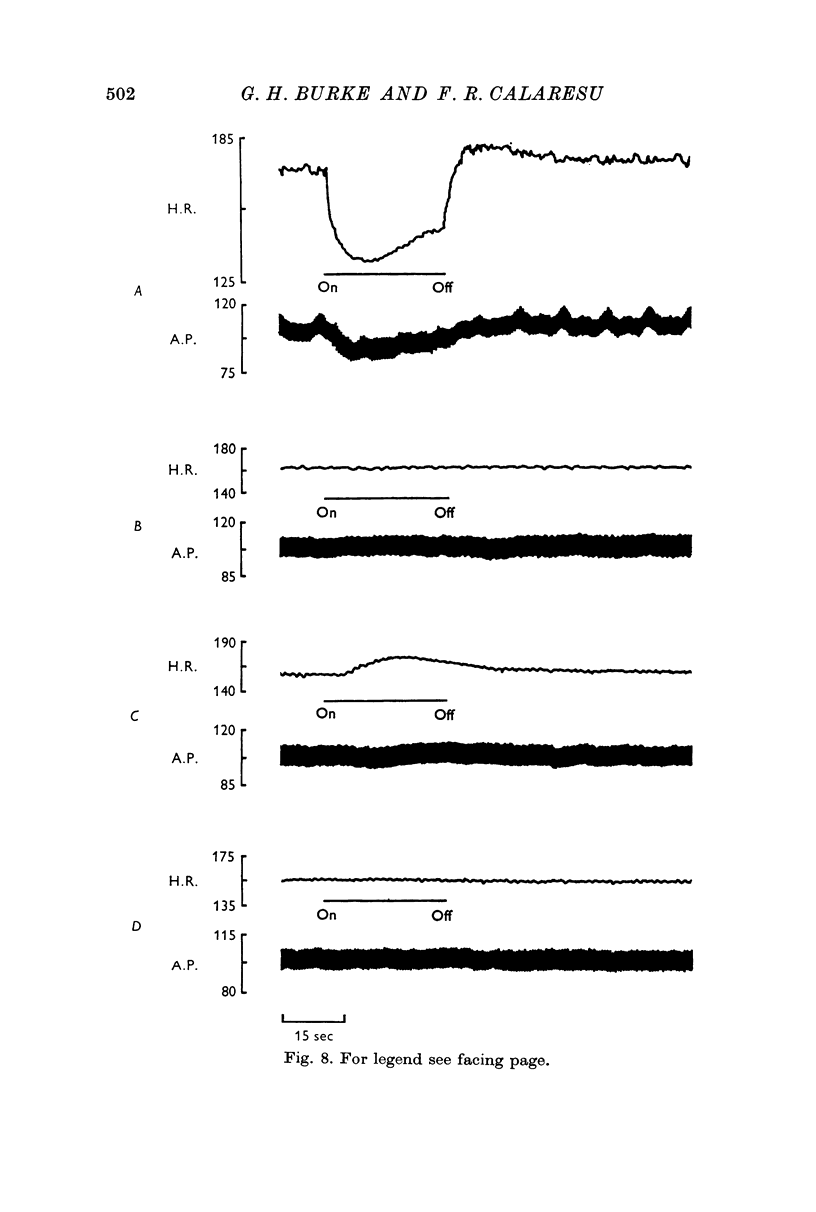
2. Postvagal tachycardia was not affected by either spinal transection at C7 or by administration of propranolol (1·5 mg/kg I.V.), but was abolished by the administration of atropine (2·0 mg/kg I.V.).
3. Postvagal tachycardia was observed to follow the vagal bradycardia induced reflexly either by administration of phenyldiguanide (100-300 μg I.V.) or by stimulation of the aortic depressor nerve.
4. In the isolated atria-vagus preparations from six rabbits a positive correlation was demonstrated between the magnitude of postvagal tachycardia and of the preceding bradycardia elicited by vagal stimulation.
5. Continuous intracellular recordings were obtained from four sinuatrial node pace-maker cells in the isolated atria-vagus preparation of the rabbit before, during and after vagal stimulation. During postvagal tachycardia the slope of the diastolic prepotential, the maximum diastolic potential, threshold potential and the overshoot were found to be increased; these changes are different from those found in pace-maker cells during adrenergic activation.
6. These findings demonstrate that postvagal tachycardia is not mediated by sympathetic adrenergic mechanisms, but suggest that it is dependent upon the preceding vagal bradycardia and may be related to an increase in net sodium influx into pace-maker cells initiated by the hyperpolarization of the pace-maker cell membrane during and immediately after vagal stimulation.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. W., Duncan W. A., Shanks R. G. Comparison of some properties of pronethalol and propranolol. Br J Pharmacol Chemother. 1965 Dec;25(3):577–591. doi: 10.1111/j.1476-5381.1965.tb01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Eccles J. C. The action of a single vagal volley on the rhythm of the heart beat. J Physiol. 1934 Sep 19;82(2):211–241. doi: 10.1113/jphysiol.1934.sp003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J. H. Release of noradrenaline from sympathetic endings. Nature. 1971 May 28;231(5300):237–240. doi: 10.1038/231237a0. [DOI] [PubMed] [Google Scholar]
- Chess G. F., Calaresu F. R. Measurement of instantaneous heart rate from the arterial pressure pulse. Med Biol Eng. 1969 Sep;7(5):571–573. doi: 10.1007/BF02551725. [DOI] [PubMed] [Google Scholar]
- Copen D. L., Cirillo D. P., Vassalle M. Tachycardia following vagal stimulation. Am J Physiol. 1968 Sep;215(3):696–703. doi: 10.1152/ajplegacy.1968.215.3.696. [DOI] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C. Circulatory and respiratory reflexes caused by aromatic guanidines. Br J Pharmacol Chemother. 1950 Mar;5(1):65–76. doi: 10.1111/j.1476-5381.1950.tb00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CARVALHO A. P., DE MELLO W. C., HOFFMAN B. F. Electrophysiological evidence for specialized fiber types in rabbit atrium. Am J Physiol. 1959 Mar;196(3):483–488. doi: 10.1152/ajplegacy.1959.196.3.483. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., SCHAUMANN W. A study of the depressor and pressor components of the cat's carotid sinus and aortic nerves using electrical stimuli of different intensities and frequencies. J Physiol. 1956 Apr 27;132(1):173–186. doi: 10.1113/jphysiol.1956.sp005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. H., Laidlaw P. P., Symons C. T. A reversed action of the vagus on the mammalian heart. J Physiol. 1910 Oct 11;41(1-2):1–18. doi: 10.1113/jphysiol.1910.sp001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK L. D., CERVONI P. Ganglionic blocking action of atropine and methylatropine. J Pharmacol Exp Ther. 1953 Dec;109(4):372–376. [PubMed] [Google Scholar]
- GLICK G., BRAUNWALD E. RELATIVE ROLES OF THE SYMPATHETIC AND PARASYMPATHETIC NERVOUS SYSTEMS IN THE REFLEX CONTROL OF HEART RATE. Circ Res. 1965 Apr;16:363–375. doi: 10.1161/01.res.16.4.363. [DOI] [PubMed] [Google Scholar]
- Green J. H., Heffron P. F. Studies upon patterns of activity in single post-ganglionic sympathetic fibres. Arch Int Pharmacodyn Ther. 1968 May;173(1):232–243. [PubMed] [Google Scholar]
- Jacobowitz D. Histochemical studies of the relationship of chromaffin cells and adrenergic nerve fibers to the cardiac ganglia of several species. J Pharmacol Exp Ther. 1967 Nov;158(2):227–240. [PubMed] [Google Scholar]
- KOTTEGODA S. R. Stimulation of isolated rabbit auricles by substances which is stimulate ganglia. Br J Pharmacol Chemother. 1953 Mar;8(1):83–86. doi: 10.1111/j.1476-5381.1953.tb00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADERS F. E. LOCAL CHOLINERGIC-ADRENERGIC INTERACTION: MECHANISM FOR THE BIPHASIC CHRONOTROPIC RESPONSE TO NERVE STIMULATION. J Pharmacol Exp Ther. 1963 Oct;142:31–38. [PubMed] [Google Scholar]
- Löffelholz K., Muscholl E. A muscarinic inhibition of the noradrenaline release evoked by postganglionic sympathetic nerve stimulation. Naunyn Schmiedebergs Arch Pharmakol. 1969;265(1):1–15. doi: 10.1007/BF01417206. [DOI] [PubMed] [Google Scholar]
- MCEWEN L. M. The effect on the isolated rabbit heart of vagal stimulation and its modification by cocaine, hexamethonium and ouabain. J Physiol. 1956 Mar 28;131(3):678–689. doi: 10.1113/jphysiol.1956.sp005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCEWEN L. M. The effect on the isolated rabbit heart of vagal stimulation and its modification by cocaine, hexamethonium and ouabain. J Physiol. 1956 Mar 28;131(3):678–689. doi: 10.1113/jphysiol.1956.sp005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu Y., Kirpekar S. M. Effects of vagal and sympathetic nerve stimulation on the isolated atria of the cat. J Pharmacol Exp Ther. 1968 Oct;163(2):330–342. [PubMed] [Google Scholar]
- OBRINK K. J., ESSEX H. E. Chronotropic effects of vagal stimulation and acetylcholine on certain mammalian hearts with special reference to the mechanism of vagal escape. Am J Physiol. 1953 Aug;174(2):321–330. doi: 10.1152/ajplegacy.1953.174.2.321. [DOI] [PubMed] [Google Scholar]
- PATHAK C. L. Alternative mechanism of cardiac acceleration in Bainbridge's infusion experiments. Am J Physiol. 1959 Aug;197:441–444. doi: 10.1152/ajplegacy.1959.197.2.441. [DOI] [PubMed] [Google Scholar]
- Raper C., Wale J. Sympathetic involvement in vagal escape and the effects of beta-receptor blocking drugs. Eur J Pharmacol. 1969 Oct;8(1):47–57. doi: 10.1016/0014-2999(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Shanks R. G. The pharmacology of beta sympathetic blockade. Am J Cardiol. 1966 Sep;18(3):308–316. doi: 10.1016/0002-9149(66)90047-6. [DOI] [PubMed] [Google Scholar]
- TODA N., FUJIWARA M. EFFECTS OF VAGAL STIMULATION ON THE TRANSMEMBRANE ACTION POTENTIALS OF ATRIUM ISOLATED FROM GUINEA PIG. Jpn J Pharmacol. 1963 Dec;13:316–317. doi: 10.1254/jjp.13.316. [DOI] [PubMed] [Google Scholar]
- TODA N., FUJIWARA M., SHIMAMOTO K. EFFECTS OF TEMPERATURE CHANGE, OXYGEN DEPRIVATION AND CATIONS ON THE ATRIAL RESPONSES TO VAGAL STIMULATION. Jpn J Pharmacol. 1964 Jun;14:118–137. doi: 10.1254/jjp.14.118. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W. Generation and conduction of impulses in the heart as affected by drugs. Pharmacol Rev. 1963 Jun;15:277–332. [PubMed] [Google Scholar]
- Toda N. Influence of sodium ions on the membrane potential of the sino-atrial node in response to sympathetic nerve stimulation. J Physiol. 1968 Jun;196(3):677–691. doi: 10.1113/jphysiol.1968.sp008529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N., Shimamoto K. The influence of sympathetic stimulation on transmembrane potentials in the S-A node. J Pharmacol Exp Ther. 1968 Feb;159(2):298–305. [PubMed] [Google Scholar]
- Vassalle M., Mandel W. J., Holder M. S. Catecholamine stores under vagal control. Am J Physiol. 1970 Jan;218(1):115–123. doi: 10.1152/ajplegacy.1970.218.1.115. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The effect of the cardiac membrane potential on the rapid availability of the sodium-carrying system. J Physiol. 1955 Jan 28;127(1):213–224. doi: 10.1113/jphysiol.1955.sp005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODBURY J. W., BRADY A. J. Intracellular recording from moving tissues with a flexibly mounted ultramicroelectrode. Science. 1956 Jan 20;123(3186):100–101. doi: 10.1126/science.123.3186.100-a. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Russell R. O., Jr Effect of combined sympathetic and vagal stimulation on heart rate in the dog. Circ Res. 1969 Apr;24(4):567–573. doi: 10.1161/01.res.24.4.567. [DOI] [PubMed] [Google Scholar]


