Abstract
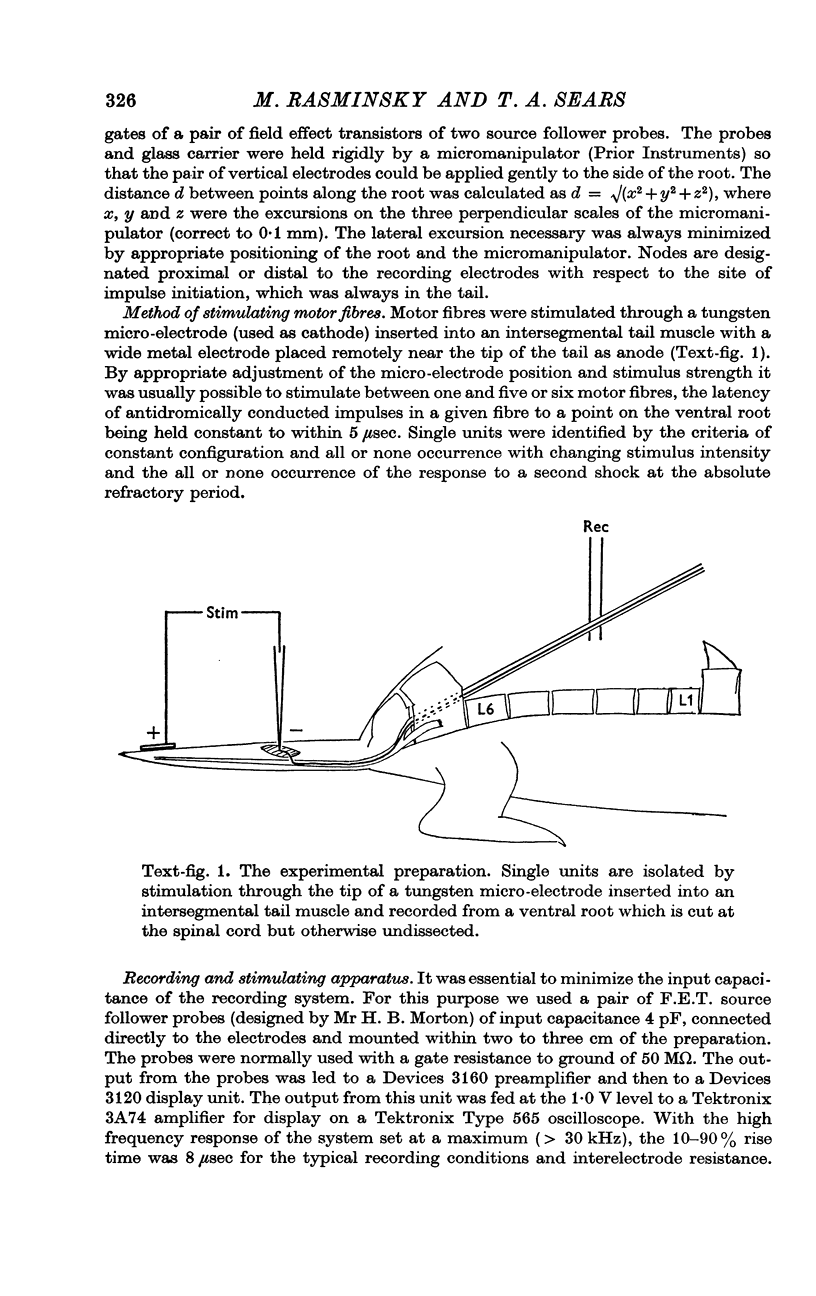
1. A new method is described for recording external longitudinal currents from single undissected nerve fibres in rat ventral roots. The method permits identification of the sites of fifteen or more successive nodes of Ranvier in a given single fibre and the measurement of internodal conduction times between them.
2. Average internodal conduction time for normal ventral root fibres of internodal length between 0·75 and 1·45 mm is 19·7 ± 4·6 (S.D.) μsec at 37° C. Internodal conduction time appeared to show a minimum for fibres of internodal length 1·0 mm.
3. Ventral roots were demyelinated by focal application of diphtheria toxin. Although conduction is markedly slowed in demyelinated fibres, sites of inward membrane current remain spatially separated indicating that conduction remains saltatory to the point of conduction block rather than becoming continuous as in unmyelinated fibres.
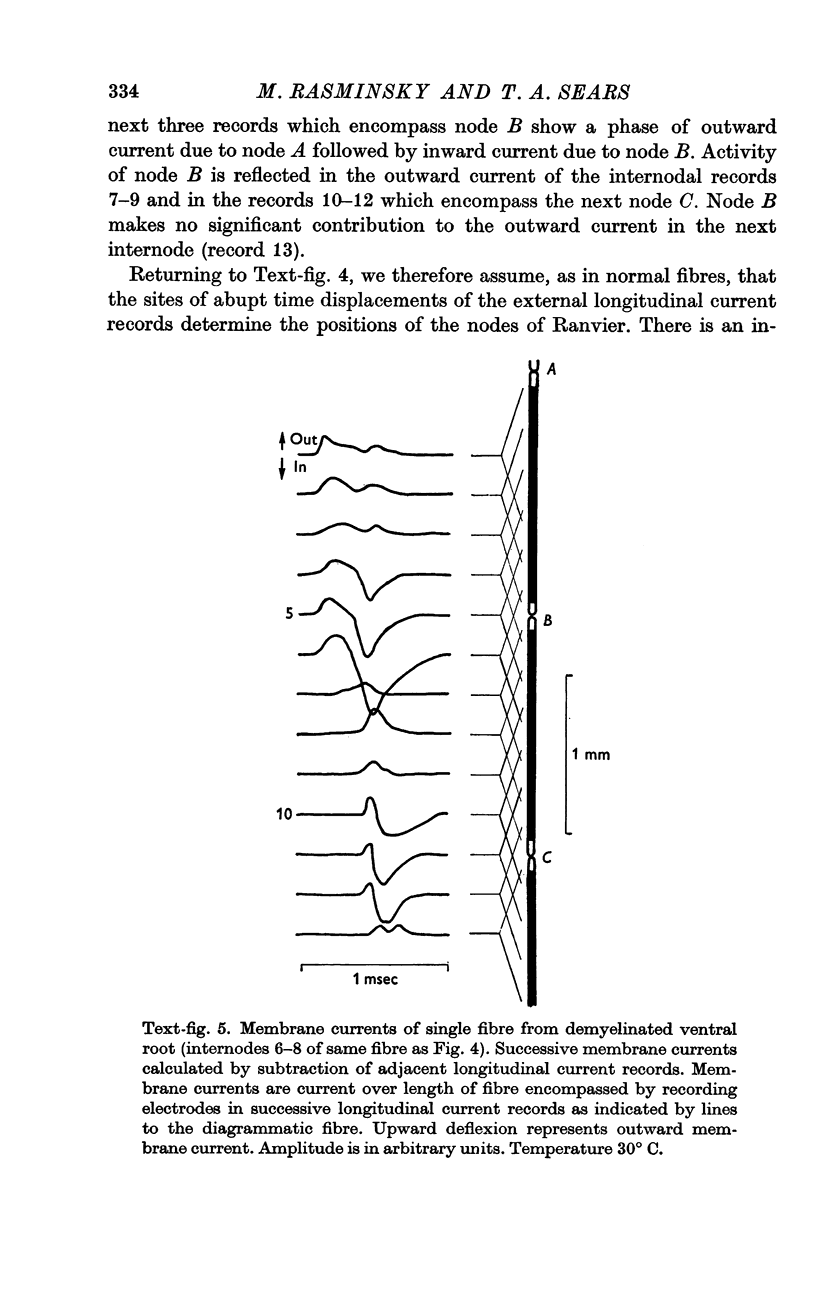
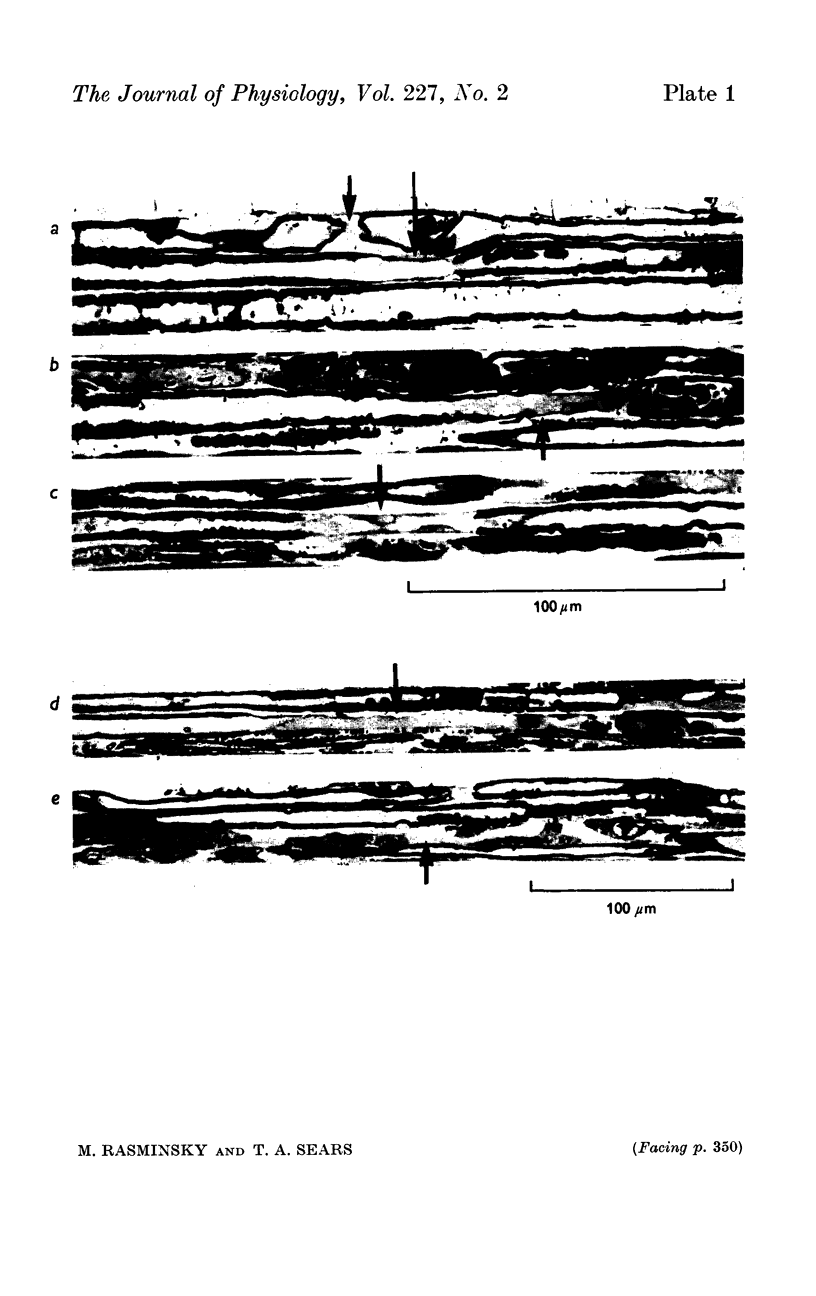
4. Slowing of conduction appears to be due to changes in the passive electrical properties of the internodal myelin. Evidence is presented suggesting that there is an increase in internodal capacitance and a decrease in internodal transverse resistance at internodes of demyelinated fibres; such changes would have the effect of delaying excitation at the nodes. The changes in passive electrical properties, which appear to be primarily in the vicinity of the nodes, would be consistent with the pathological changes observed in demyelinated fibres.
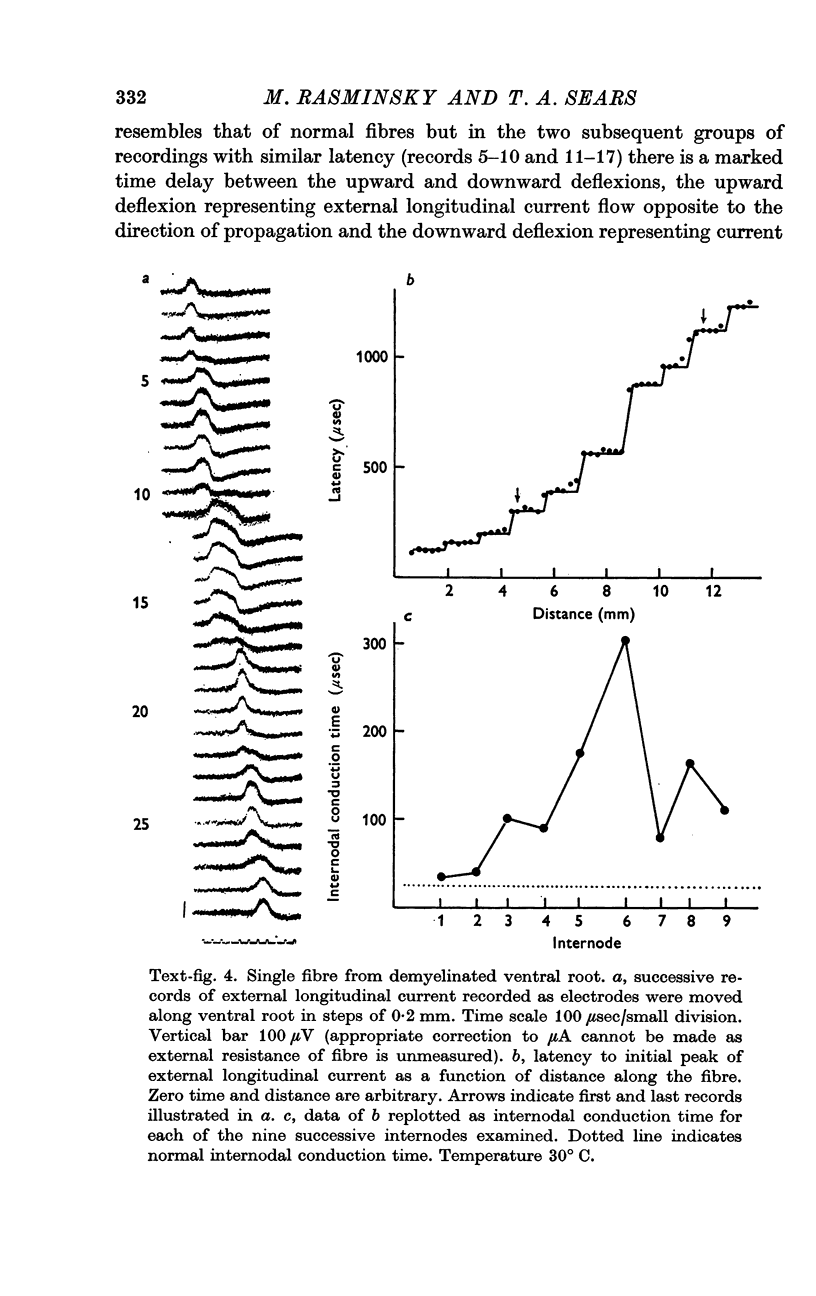
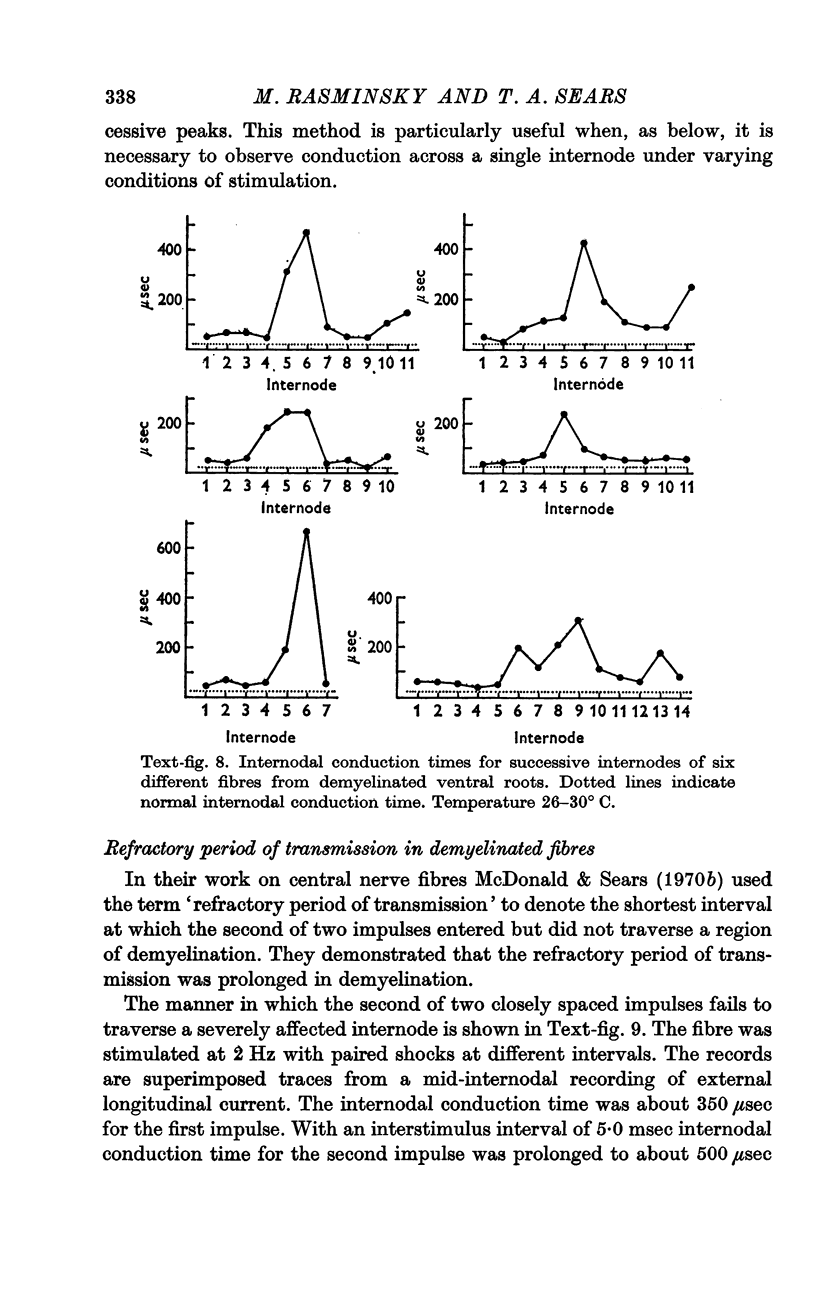
5. Internodal conduction times in demyelinated fibres have ranged from normal (26 μsec at 30° C) to more than 600 μsec. There is a great variation in internodal conduction time at successive internodes of a given single fibre; this presumably reflects the varying severity of demyelination of successive internodes.
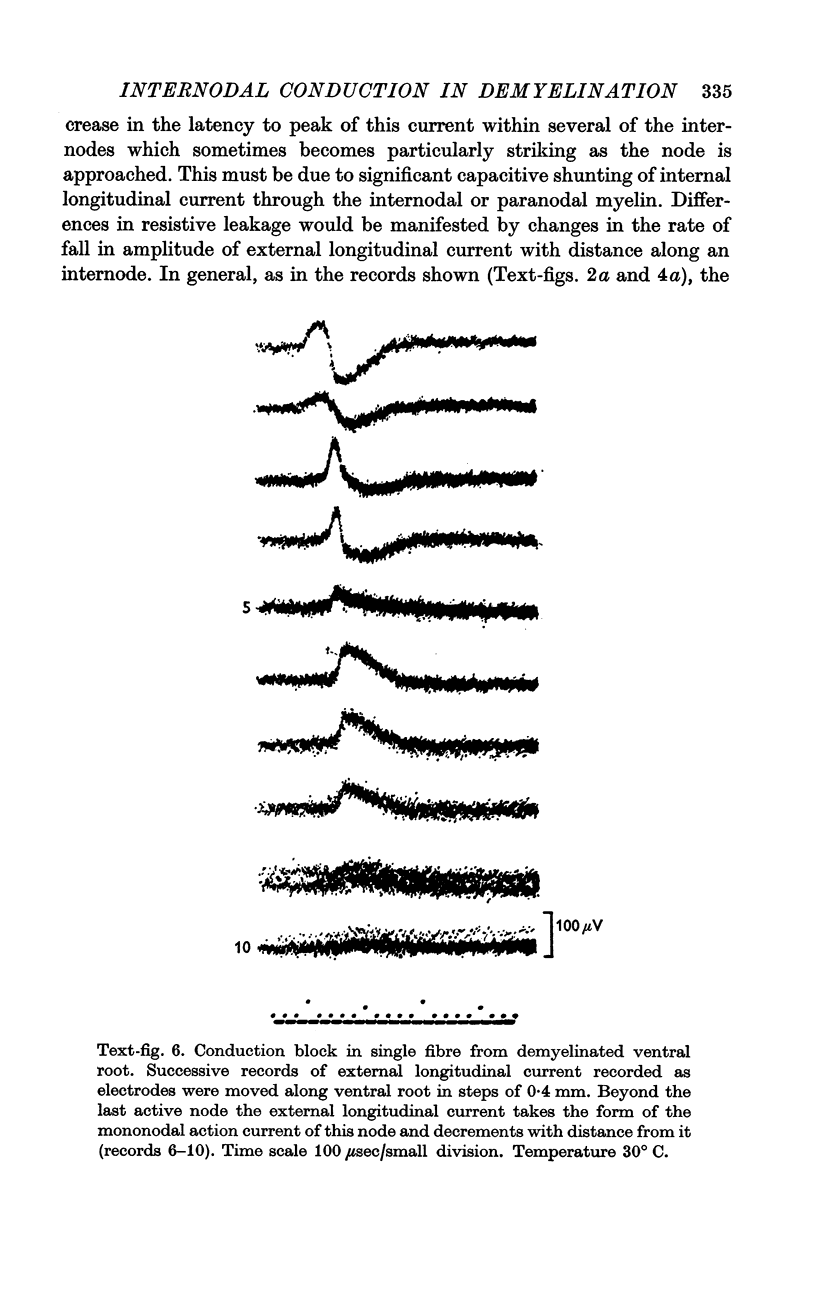
6. As in normal fibres, nodes of demyelinated fibres generate less current when excited by the second of two closely spaced impulses. This results in an increased internodal conduction time for the second impulse and, at a critically short interstimulus interval, conduction block of the second impulse.
7. The increased refractory period of transmission of internodes with increased internodal conduction times is a consequence of the decreased ability of such internodes to sustain propagation in the face of small decreases in nodal current.
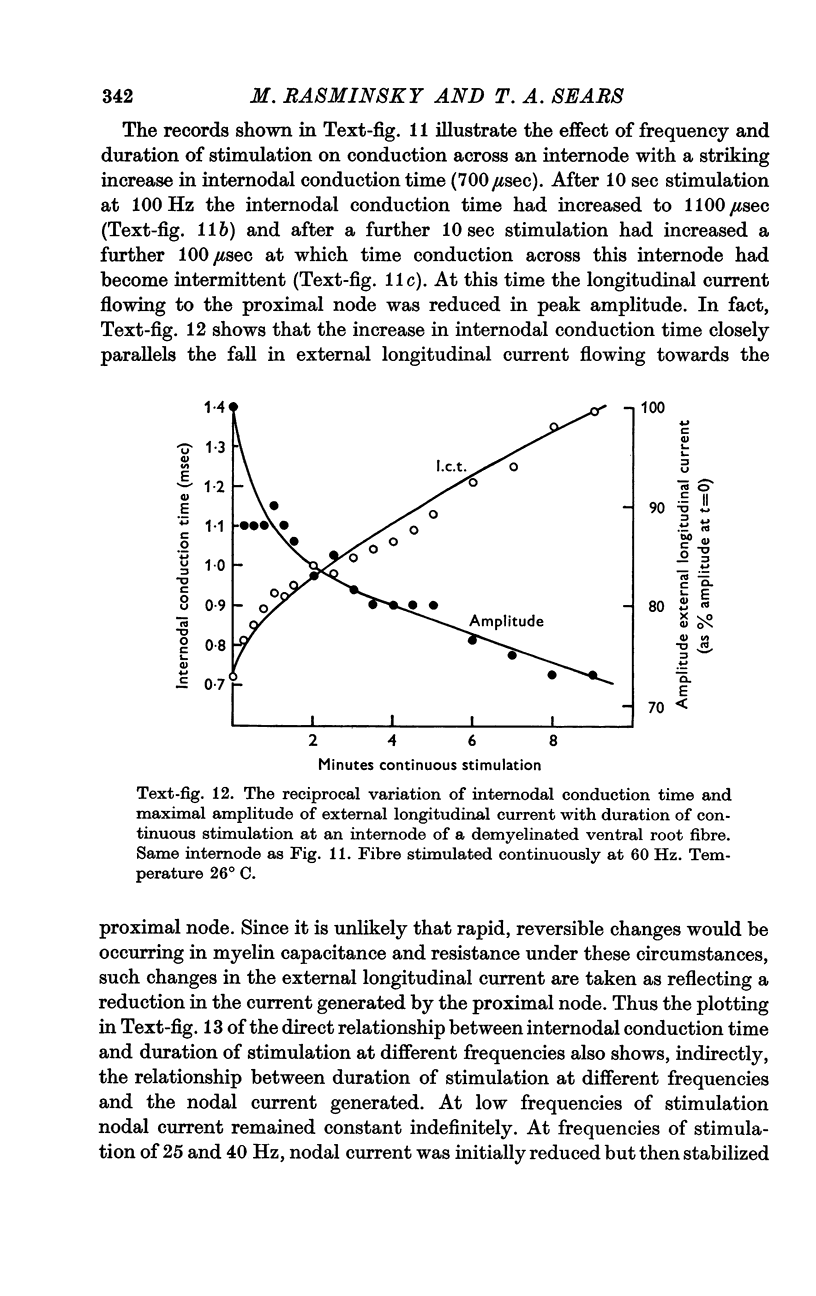
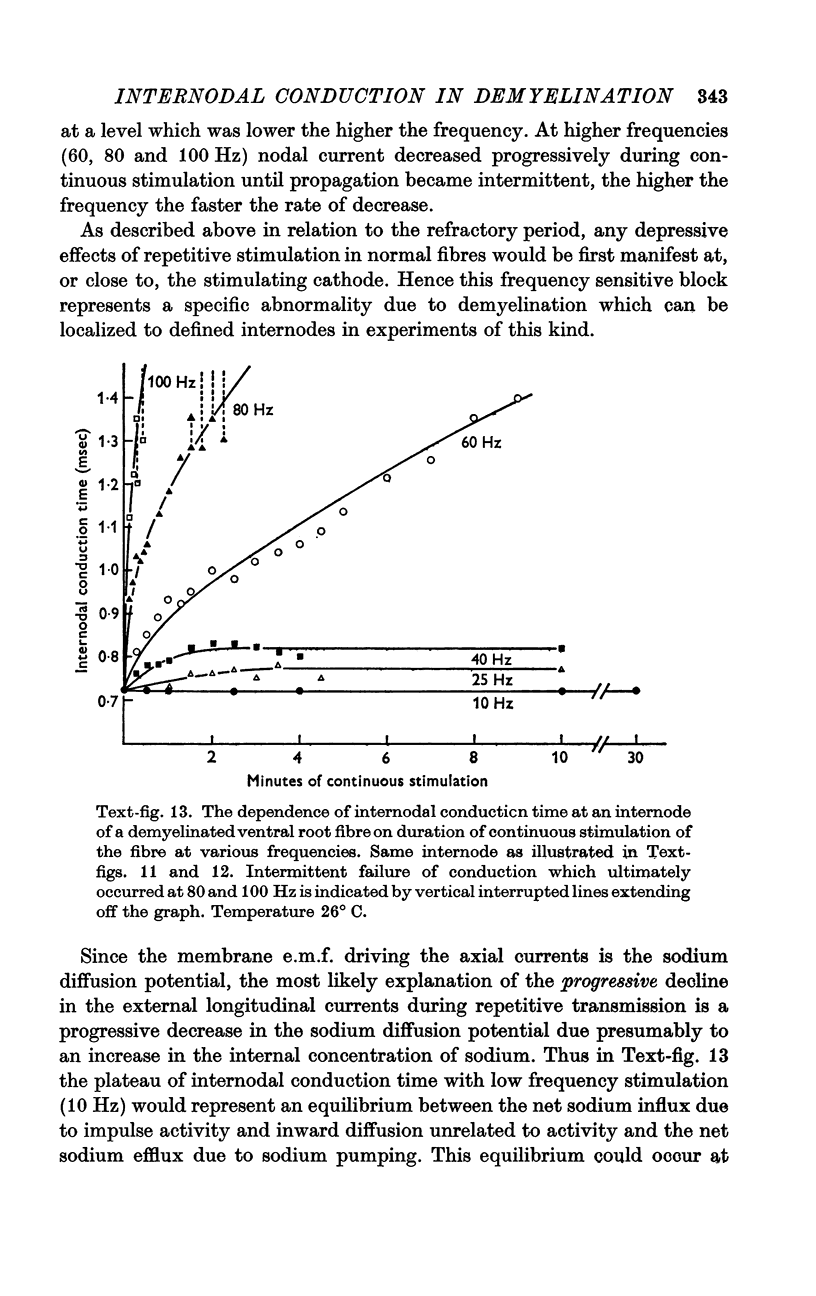
8. During tetanic stimulation, increases in internodal conduction time are associated with corresponding decreases in nodal current generated by the node proximal to the internode in question.
9. It is suggested that changes in the magnitude of the nodal current during repetitive activity are due to changes in transmembrane concentration gradients of sodium, the increased internodal conduction time and eventual conduction block during tetanic stimulation being caused by intracellular sodium accumulation.
10. Intracellular sodium accumulation is also offered as the explanation for the post-tetanic depression seen in demyelinated fibres.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allt G., Cavanagh J. B. Ultrastructural changes in the region of the node of ranvier in the rat caused by diphtheria toxin. Brain. 1969;92(2):459–468. doi: 10.1093/brain/92.2.459. [DOI] [PubMed] [Google Scholar]
- Ballin R. H., Thomas P. K. Electron microscope observations on demyelination and remyelination in experimental allergic neuritis. I. Demyelination. J Neurol Sci. 1969 Jan-Feb;8(1):1–18. doi: 10.1016/0022-510x(69)90037-9. [DOI] [PubMed] [Google Scholar]
- Bergman C. Increase of sodium concentration near the inner surface of the nodal membrane. Pflugers Arch. 1970;317(4):287–302. doi: 10.1007/BF00586578. [DOI] [PubMed] [Google Scholar]
- Berthold C. H., Skoglund S. Histochemical and ultrastructural demonstration of mitochondria in the paranodal region of developing feline spinal roots and nerves. Acta Soc Med Ups. 1967;72(1):37–70. [PubMed] [Google Scholar]
- Berthold C. H. Ultrastructure of the node-paranode region of mature feline ventral lumbar spinal-root fibres. Acta Soc Med Ups. 1968;73(Suppl):37–70. [PubMed] [Google Scholar]
- CAVANAGH J. B., JACOBS J. M. SOME QUANTITATIVE ASPECTS OF DIPHTHERITIC NEUROPATHY. Br J Exp Pathol. 1964 Jun;45:309–322. [PMC free article] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. CHANGES IN NERVE CONDUCTION IN EXPERIMENTAL ALLERGIC NEURITIS. J Neurol Neurosurg Psychiatry. 1964 Apr;27:106–115. doi: 10.1136/jnnp.27.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin C. M., Jack J. J. Internodal length and conduction velocity of cat muscle afferent nerve fibres. J Physiol. 1972 Apr;222(1):92P–93P. [PubMed] [Google Scholar]
- Davis F. A. Impairment of repetitive impulse conduction in experimentally demyelinated and pressure-injured nerves. J Neurol Neurosurg Psychiatry. 1972 Aug;35(4):537–544. doi: 10.1136/jnnp.35.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck P. J., Lais A. C. Electron microscopy of teased nerve fibers: method permitting examination of repeating structures of same fiber. Brain Res. 1970 Oct 28;23(3):418–424. doi: 10.1016/0006-8993(70)90067-3. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol. 1949 May 15;108(3):315–339. [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. M. Experimental diphtheritic neuropathy in the rat. Br J Exp Pathol. 1967 Apr;48(2):204–216. [PMC free article] [PubMed] [Google Scholar]
- Koles Z. J., Rasminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):351–364. doi: 10.1113/jphysiol.1972.sp010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUSSIER J. J., RUSHTON W. A. H. The excitability of a single fiber in a nerve trunk. J Physiol. 1952 May;117(1):87–108. [PMC free article] [PubMed] [Google Scholar]
- Lehmann H. J., Lehmann G., Tackmann W. Refraktärperiode und Ubermittlung von Serienimpulsen im N. tibialis des Meerschweinchens bei experimenteller allergischer Neuritis. Z Neurol. 1971 Apr 28;199(1):67–85. [PubMed] [Google Scholar]
- Lehmann H. J., Pretschner D. P. Experimentelle Untersuchungen zum Engpasssyndrom peripherer Nerven. Dtsch Z Nervenheilkd. 1966 May 6;188(4):308–330. [PubMed] [Google Scholar]
- Lehmann H. J., Tackmann W. Die Ubermittlung frequenter Impulsserien in demyelinisierten und in degenerierenden Nervenfasern. Arch Psychiatr Nervenkr (1970) 1970;213(3):215–227. doi: 10.1007/BF00342658. [DOI] [PubMed] [Google Scholar]
- Lehmann H. J., Tackmann W., Lehmann G. Funktionsänderung markhaltiger Nervenfasern im N. tibialis des Meerschweinchens bei postdiphtherischer Polyneuritis. Z Neurol. 1971 Apr 28;199(1):86–104. [PubMed] [Google Scholar]
- MAYER R. F., DENNY-BROWN D. CONDUCTION VELOCITY IN PERIPHERAL NERVE DURING EXPERIMENTAL DEMYELINATION IN THE CAT. Neurology. 1964 Aug;14:714–726. doi: 10.1212/wnl.14.8_part_1.714. [DOI] [PubMed] [Google Scholar]
- MCDONALD W. I. THE EFFECTS OF EXPERIMENTAL DEMYELINATION ON CONDUCTION IN PERIPHERAL NERVE: A HISTOLOGICAL AND ELECTROPHYSIOLOGICAL STUDY. I. CLINICAL AND HISTOLOGICAL OBSERVATIONS. Brain. 1963 Sep;86:481–500. doi: 10.1093/brain/86.3.481. [DOI] [PubMed] [Google Scholar]
- MCDONALD W. I. THE EFFECTS OF EXPERIMENTAL DEMYELINATION ON CONDUCTION IN PERIPHERAL NERVE: A HISTOLOGICAL AND ELECTROPHYSIOLOGICAL STUDY. II. ELECTROPHYSIOLOGICAL OBSERVATIONS. Brain. 1963 Sep;86:501–524. doi: 10.1093/brain/86.3.501. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Sears T. A. Effect of a demyelinating lesion on conduction in the central nervous system studied in single nerve fibres. J Physiol. 1970 Apr;207(2):53P–54P. [PubMed] [Google Scholar]
- McDonald W. I., Sears T. A. Effect of demyelination on conduction in the central nervous system. Nature. 1969 Jan 11;221(5176):182–183. doi: 10.1038/221182a0. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Sears T. A. The effects of experimental demyelination on conduction in the central nervous system. Brain. 1970;93(3):583–598. doi: 10.1093/brain/93.3.583. [DOI] [PubMed] [Google Scholar]
- McDonald W. I. Structural and functional changes in human and experimental neuropathy. Mod Trends Neurol. 1967;4(0):145–164. [PubMed] [Google Scholar]
- Morgan-Hughes J. A. Experimental diphtheritic neuropathy. A pathological and electrophysiological study. J Neurol Sci. 1968 Jul-Aug;7(1):157–175. doi: 10.1016/0022-510x(68)90011-7. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasminsky M., Sears T. A. Internodal conduction in normal and demyelinated mammalian single nerve fibres. J Physiol. 1971;217 (Suppl):66P–67P. [PubMed] [Google Scholar]
- Spencer P. S., Thomas P. K. The examination of isolated nerve fibres by light and electron microscopy, with observations on demyelination proximal to neuromas. Acta Neuropathol. 1970;16(3):177–186. doi: 10.1007/BF00687357. [DOI] [PubMed] [Google Scholar]
- WEBSTER H. D., SPIRO D., WAKSMAN B., ADAMS R. D. Phase and electron microscopic studies of experimental demyelination. II. Schwann cell changes in guinea pig sciatic nerves during experimental diphtheritic neuritis. J Neuropathol Exp Neurol. 1961 Jan;20:5–34. doi: 10.1097/00005072-196101000-00002. [DOI] [PubMed] [Google Scholar]
- WILLIAMS P. L., LANDON D. N. Paranodal apparatus of peripheral myelinated nerve fibres of mammals. Nature. 1963 May 18;198:670–673. doi: 10.1038/198670a0. [DOI] [PubMed] [Google Scholar]



