Abstract
1. The carbamate (HbCO2) concentration in oxygenated and deoxygenated human adult and foetal red blood cells was estimated at a constant pressure of carbon dioxide (PCO2 = 40 mm Hg) and various pH values of the serum. The Donnan ratio for chloride and bicarbonate ions was used to calculate the bicarbonate concentration in the red cells. With this figure the carbamate concentration was calculated as follows:
[HbCO2] = [Total CO2] - [HCO-3] - [dissolved CO2].
2. At a given pH value in the red cell deoxygenated foetal red cells contain more HbCO2 than deoxygenated adult ones. Upon oxygenation (at constant pH) HbCO2 drops in both types of erythrocytes to lower values than in deoxygenated cells. The fraction of `oxylabile carbamate' (-ΔHbCO2/ΔHbO2) at a red cell pH of 7·2 and a PCO2 of 40 mm Hg is 0·117 in foetal and 0·081 in adult erythrocytes.
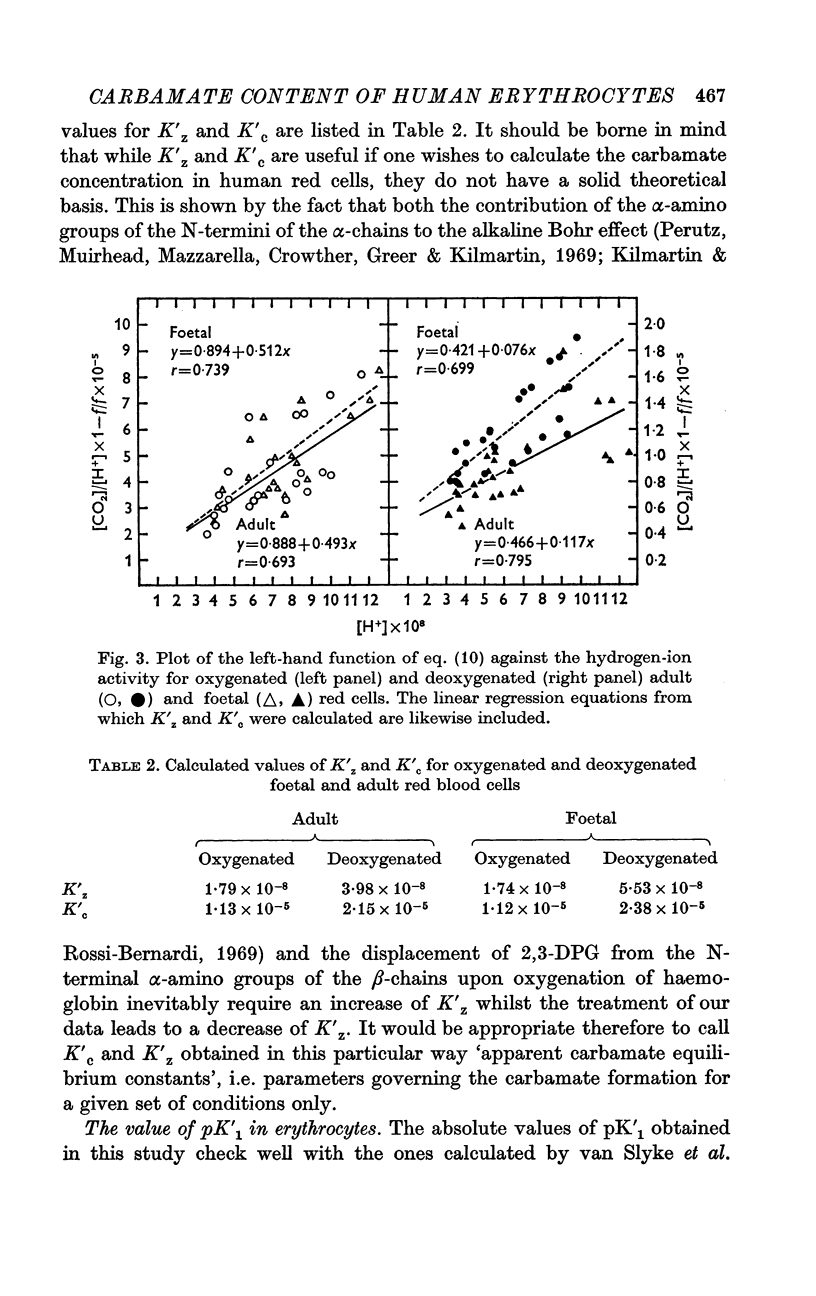
3. From the fraction of moles carbamate formed per Hb monomer (moles CO2/mole Hbi) K′c and K′z, the apparent carbamate equilibrium constants were calculated which can be used to estimate the carbamate concentration in normal adult and foetal blood.
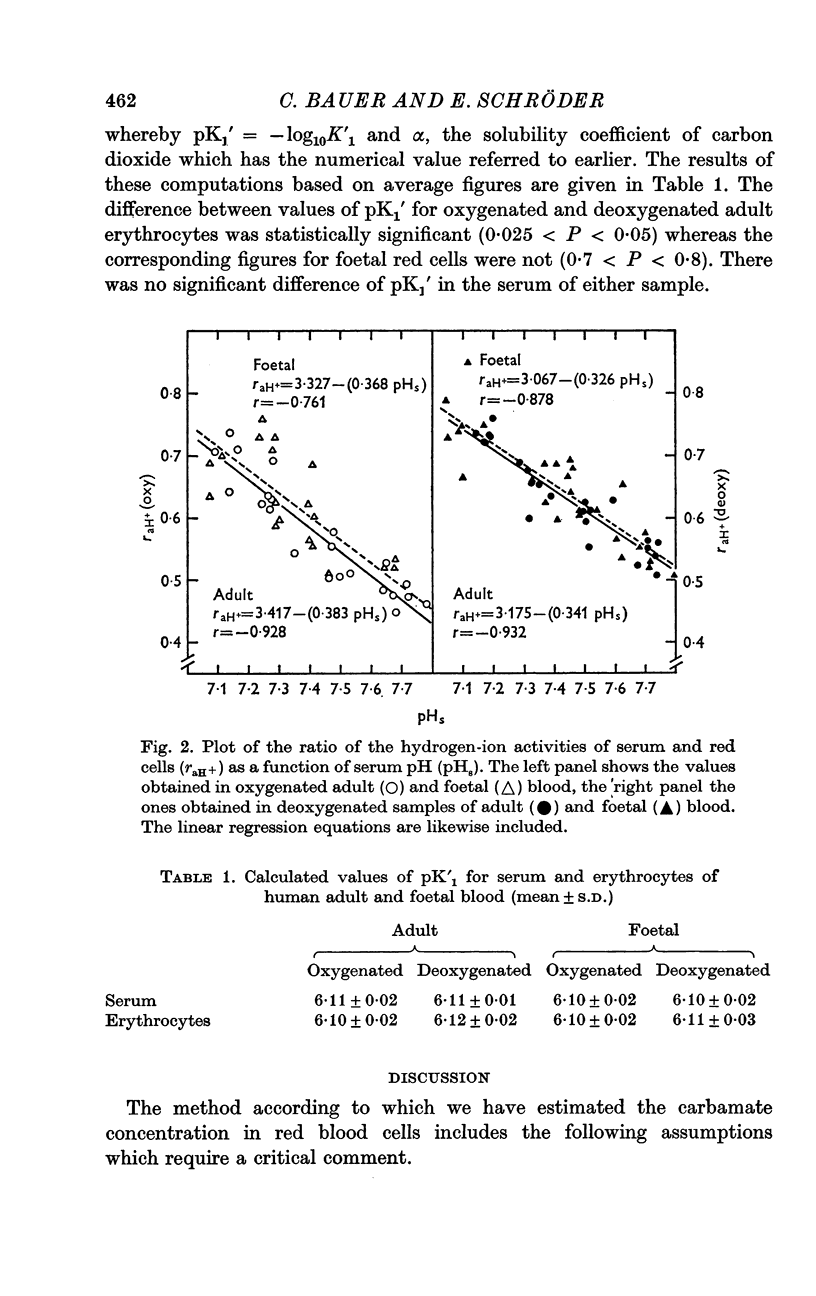
4. The first apparent dissociation constant of carbonic acid is significantly higher in oxygenated (-log10K′1 = pK′1 = 6·10) than in deoxygenated (pK′1 = 6·12) adult red cells, whereas in foetal red cells the difference is smaller and statistically not significant.
5. For a given set of physiological conditions in arterial and mixed venous blood in respect to oxygen saturation, PCO2 and pH, the fractional contribution of carbamino compounds of haemoglobin to the amount of carbon dioxide which is exchanged during the respiratory cycle was computed on the basis of the present results and found to be 10·5% in adult and 19% in foetal blood.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BRUNORI M., FRONTICELLI C., BUCCI E., REICHLIN M., FANELLI A. R. THE OXYGEN BOHR EFFECT OF HUMAN FETAL HEMOGLOBIN. Arch Biochem Biophys. 1964 Dec;108:569–572. doi: 10.1016/0003-9861(64)90443-6. [DOI] [PubMed] [Google Scholar]
- BARTELS H., WRBITZKY R. [Determination of carbon dioxide absorption coefficients between 15 and 38 degrees C. in water and plasma]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:162–168. [PubMed] [Google Scholar]
- Bauer C. Antagonistic influence of Co2 and 2,3 diphosphoglycerate on the Bohr effect of human haemoglobin. Life Sci. 1969 Oct 15;8(20):1041–1046. doi: 10.1016/0024-3205(69)90455-x. [DOI] [PubMed] [Google Scholar]
- Bauer C. Reduction of the carbon dioxide affinity of human haemoglobin solutions by 2,3 diphosphoglycerate. Respir Physiol. 1970 Jul;10(1):10–19. doi: 10.1016/0034-5687(70)90022-8. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSBY W. H., FURTH F. W. A modification of the benzidine method for measurement of hemoglobin in plasma and urine. Blood. 1956 Apr;11(4):380–383. [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- DEANE N., SMITH H. W. The apparent first dissociation constant, pK1, of carbonic acid in the human erythrocyte. J Biol Chem. 1957 Jul;227(1):101–106. [PubMed] [Google Scholar]
- Ferguson J. K. Carbamino compounds of CO(2) with human haemoglobin and their role in the transport of CO(2). J Physiol. 1936 Oct 16;88(1):40–55. doi: 10.1113/jphysiol.1936.sp003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R. E., Constantine H. P., Craw M. R., Rotman H. H., Klocke R. A. Reaction of CO2 with human hemoglobin solution. J Biol Chem. 1968 Jun 25;243(12):3317–3326. [PubMed] [Google Scholar]
- HILPERT P., FLEISCHMANN R. G., KEMPE D., BARTELS H. THE BOHR EFFECT RELATED TO BLOOD AND ERYTHROCYTE PH. Am J Physiol. 1963 Aug;205:337–340. doi: 10.1152/ajplegacy.1963.205.2.337. [DOI] [PubMed] [Google Scholar]
- JACKSON D. M., NUTT M. E. Intercellular plasma and its effect on absolute red cell volume determination. J Physiol. 1951 Oct 29;115(2):196–205. doi: 10.1113/jphysiol.1951.sp004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay A. W., Burton A. C. Direct measurement of potential difference across the human red blood cell membrane. Biophys J. 1969 Feb;9(2):115–121. doi: 10.1016/S0006-3495(69)86372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke R. A. Influence of oxygenation, pH, and 2, 3-diphosphyglycerate on carbon dioxide exchange. Chest. 1972 Feb;61(2 Suppl):20S–22S. doi: 10.1378/chest.61.2_supplement.20s. [DOI] [PubMed] [Google Scholar]
- LAUE D. Ein neues Tonometer zur raschen Aquilibrierung von Blut mit verschiedenen Gasdrucken. Pflugers Arch. 1951 Dec;254(2):142–143. doi: 10.1007/BF00369991. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Riggs A. Mechanism of the enhancement of the Bohr effect in mammalian hemoglobins by diphosphoglycerate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2062–2065. doi: 10.1073/pnas.68.9.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Bernardi L., Roughton F. J. The specific influence of carbon dioxide and carbamate compounds on the buffer power and Bohr effects in human haemoglobin solutions. J Physiol. 1967 Mar;189(1):1–29. doi: 10.1113/jphysiol.1967.sp008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton F. J. Some recent work on the interactions of oxygen, carbon dioxide and haemoglobin. Biochem J. 1970 May;117(5):801–812. doi: 10.1042/bj1170801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggaard-Andersen O. Oxygen-linked hydrogen ion binding of human hemoglobin. Effects of carbon dioxide and 2,3-diphosphoglycerate. I. Studies on erythrolysate. Scand J Clin Lab Invest. 1971 Jun;27(4):351–360. doi: 10.3109/00365517109080230. [DOI] [PubMed] [Google Scholar]
- Tomita S., Riggs A. Studies of the interaction of 2,3-diphosphoglycerate and carbon dioxide with hemoglobins from mouse, man, and elephant. J Biol Chem. 1971 Feb 10;246(3):547–554. [PubMed] [Google Scholar]
- de Bruin S. H., Janssen L. H., van Os G. A. Effect of 2,3-diphosphoglycerate on the Bohr effect of human adult hemoglobin. Biochem Biophys Res Commun. 1971 Oct 15;45(2):544–550. doi: 10.1016/0006-291x(71)90854-0. [DOI] [PubMed] [Google Scholar]
- de Verdier C. H., Garby L. Low binding of 2,3-diphosphoglycerate to haemoglobin F. A contribution to the knowledge of the binding site and an explanation for the high oxygen affinity of foetal blood. Scand J Clin Lab Invest. 1969 Apr;23(2):149–151. doi: 10.3109/00365516909077018. [DOI] [PubMed] [Google Scholar]


