Abstract
1. Intracellular and extracellular electrodes were used to study spontaneous and impulse-linked release of transmitter at locust retractor unguis nerve-muscle synapses.
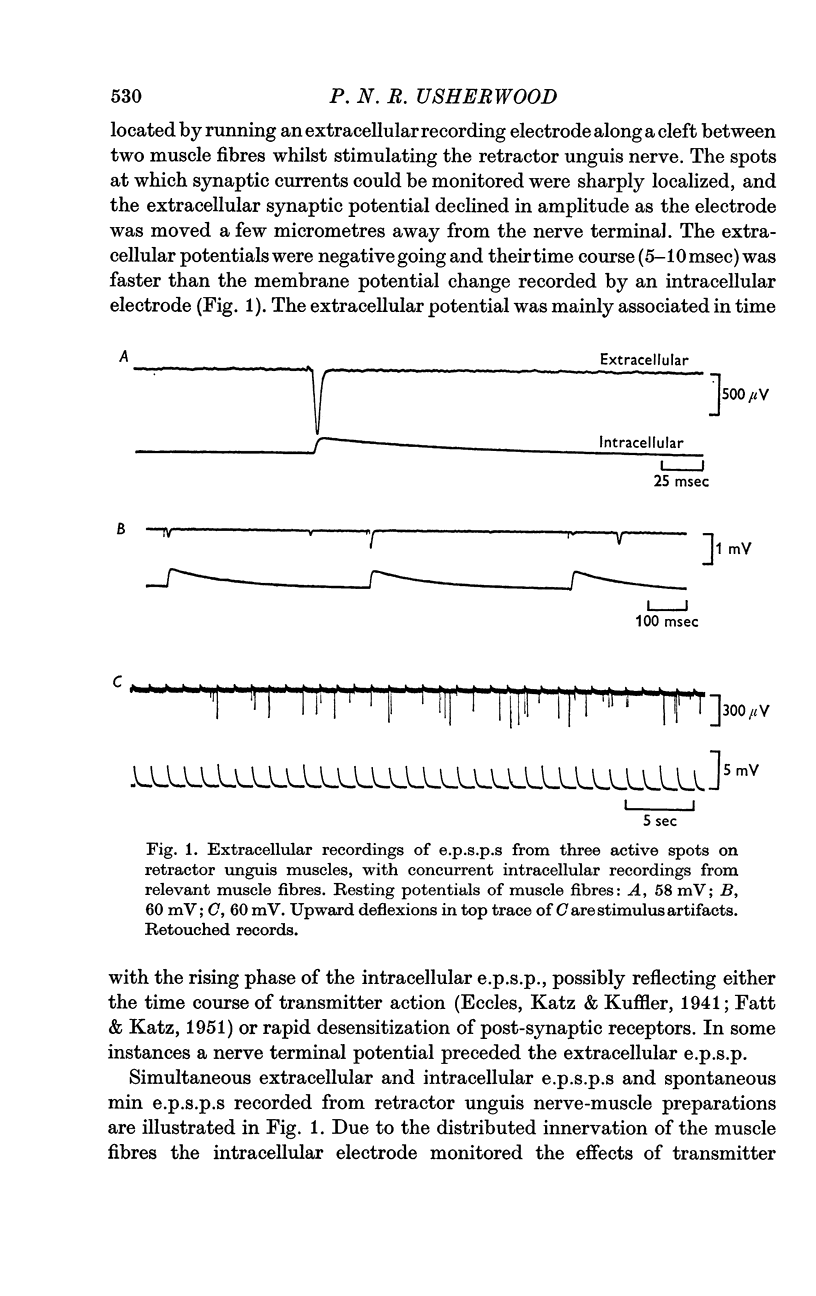
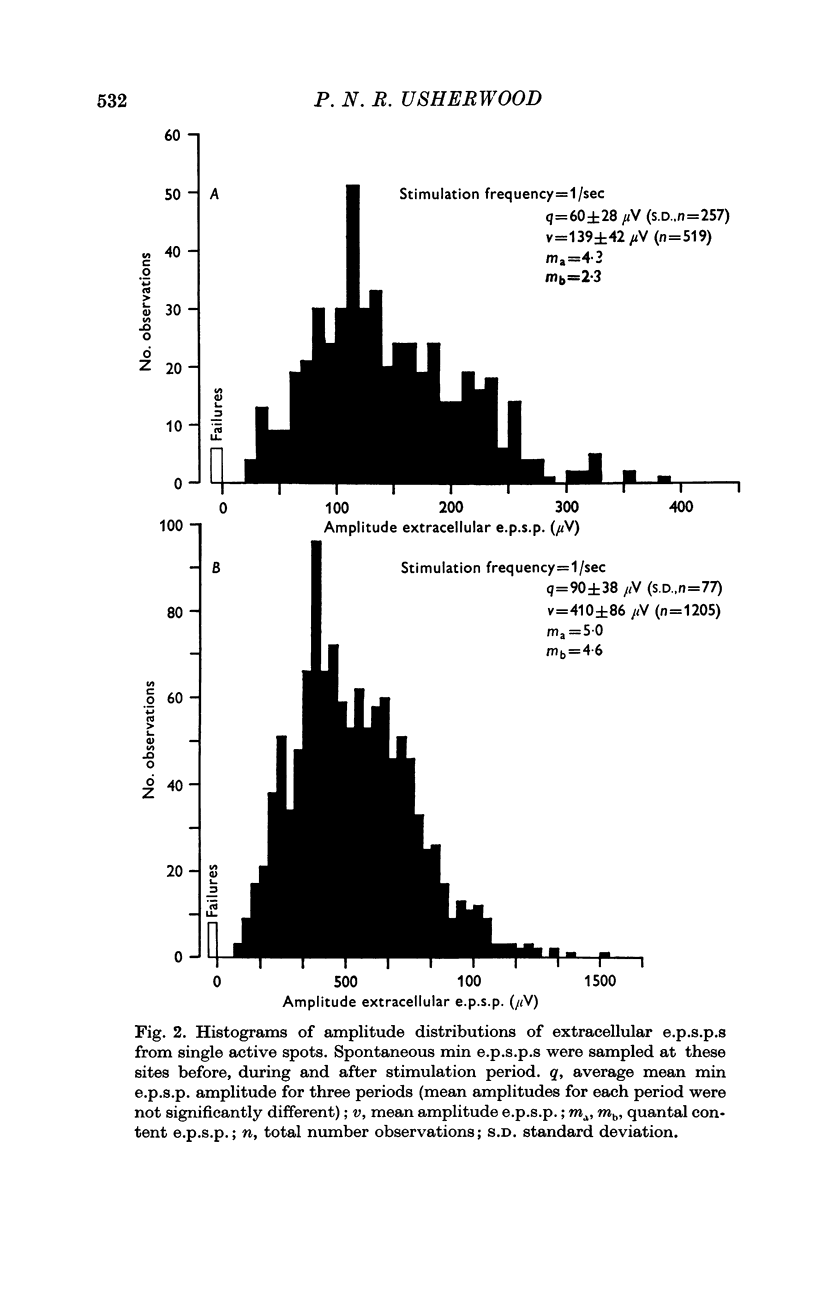
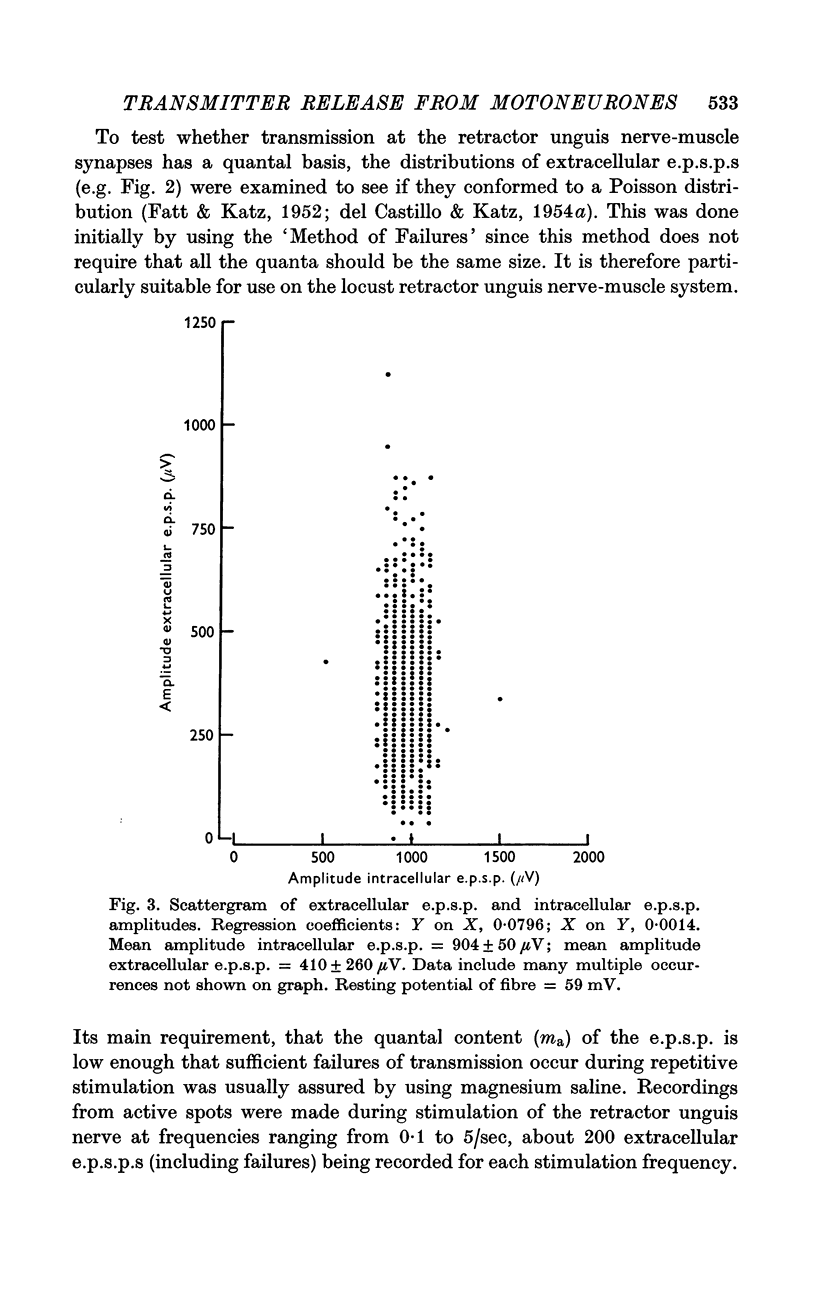
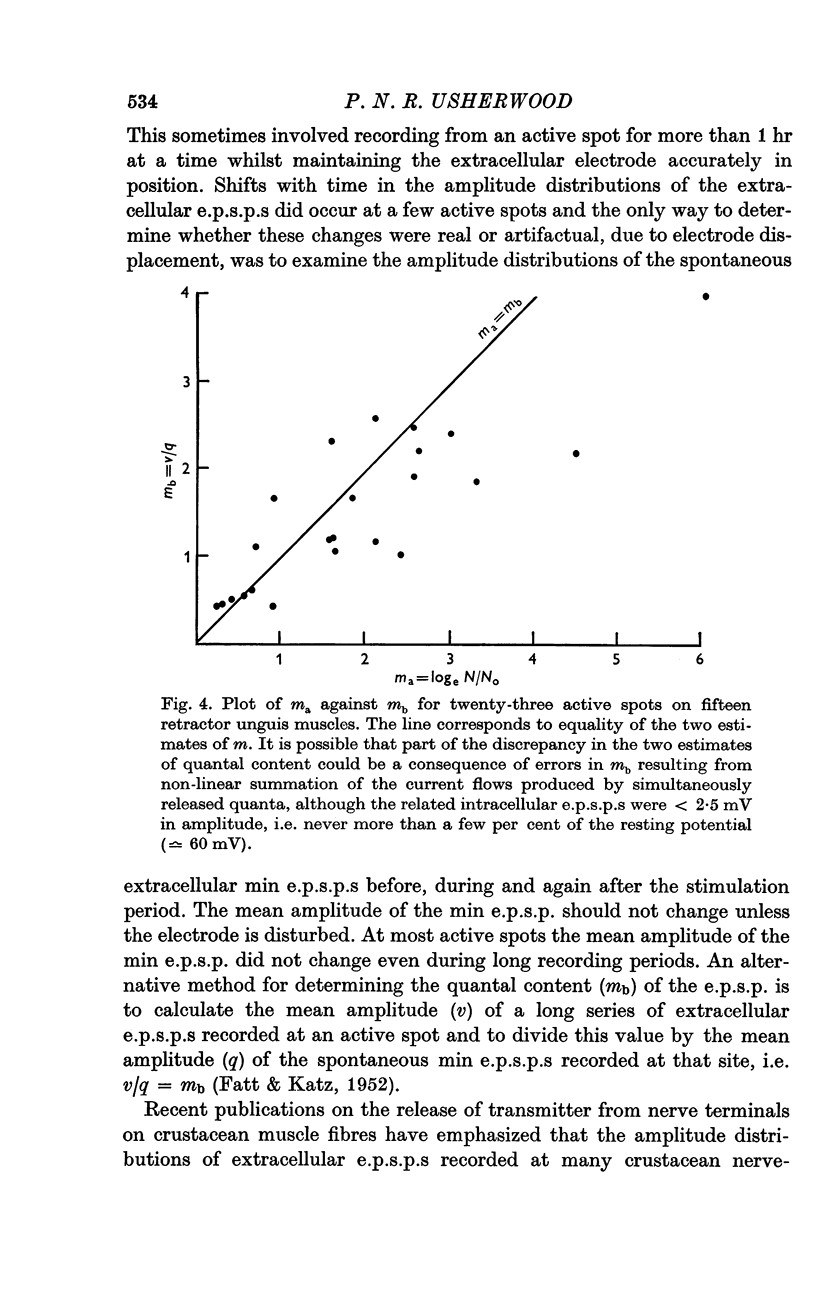
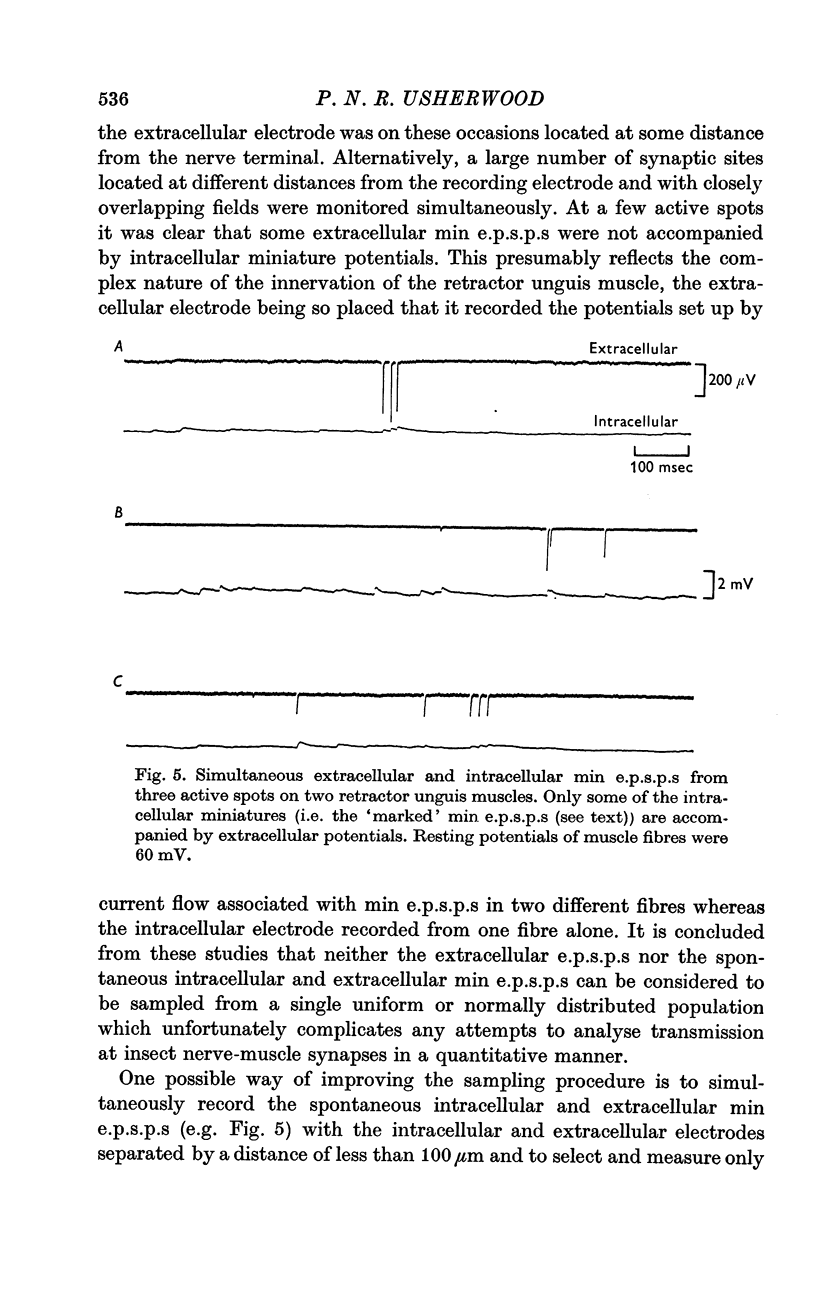
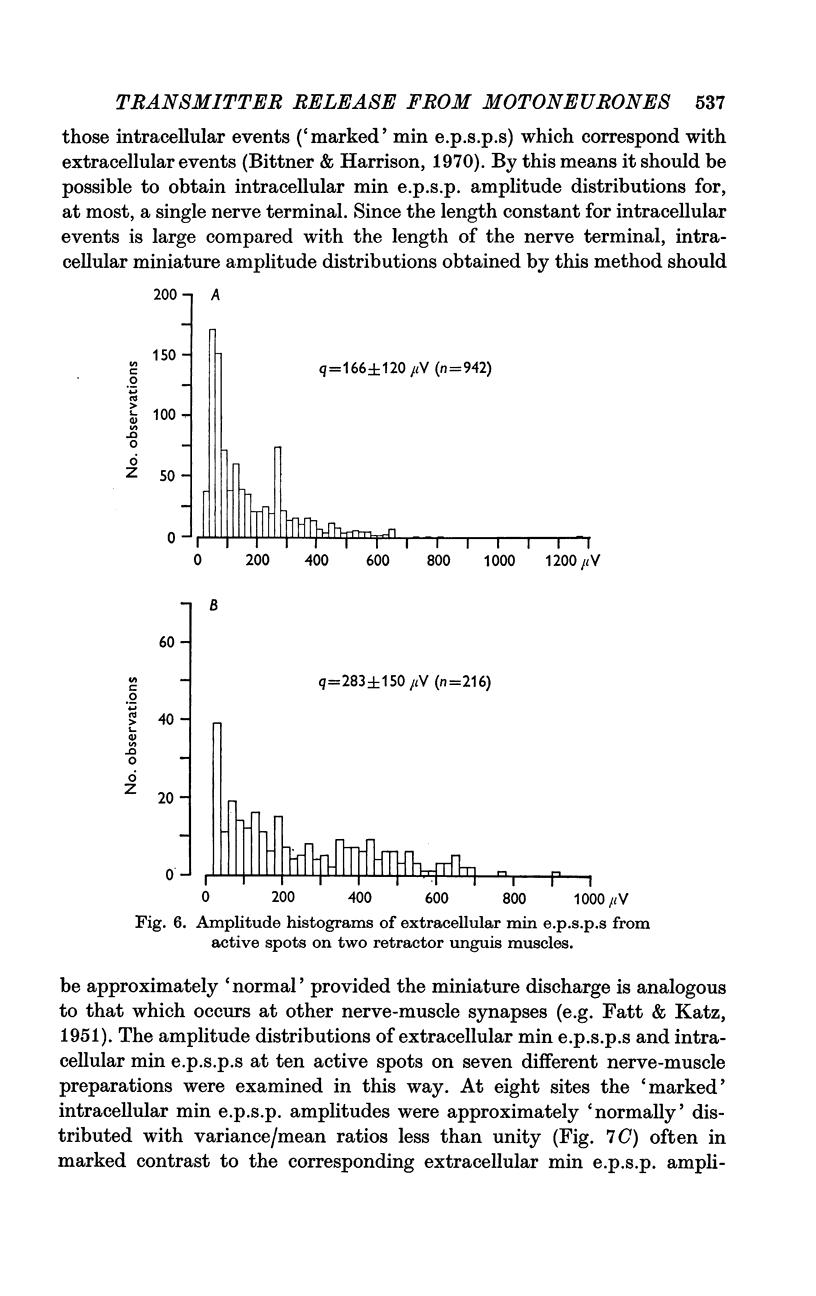
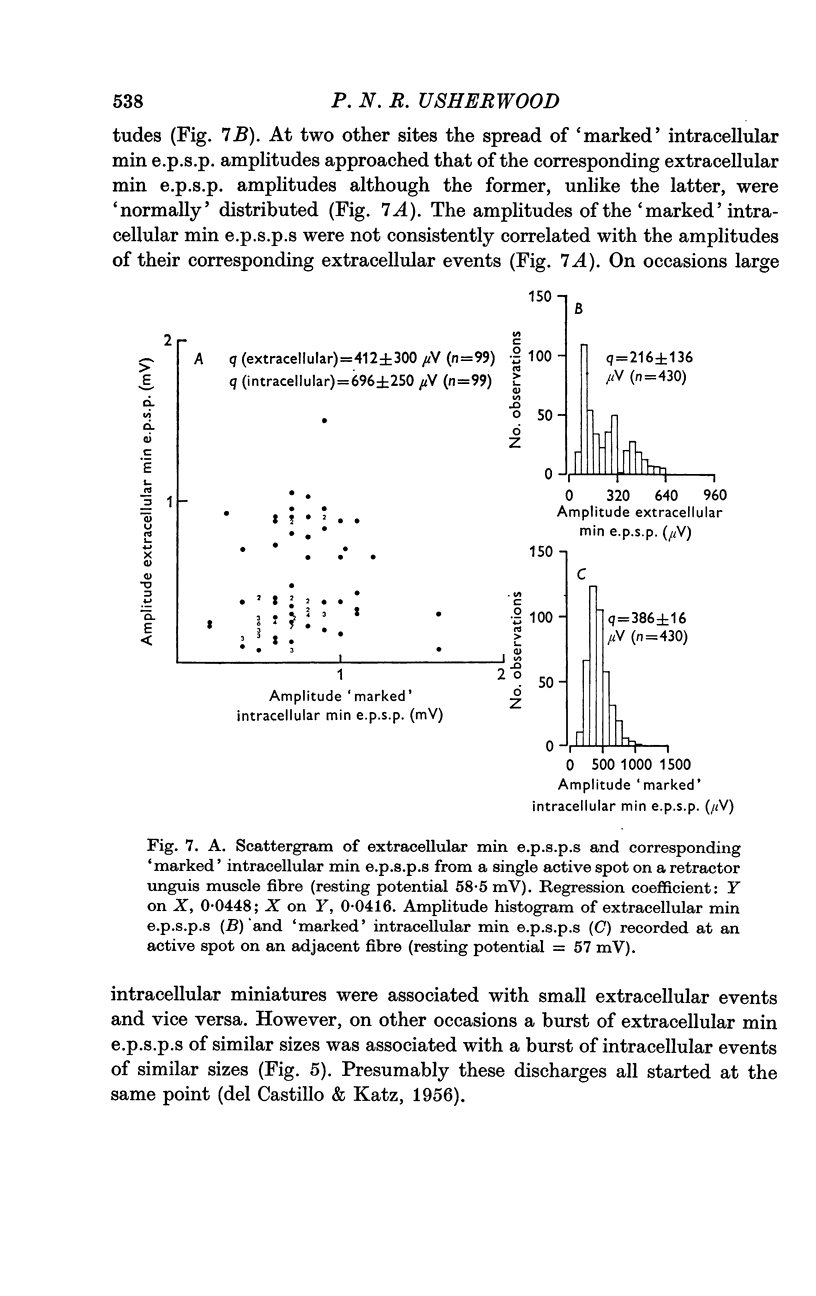
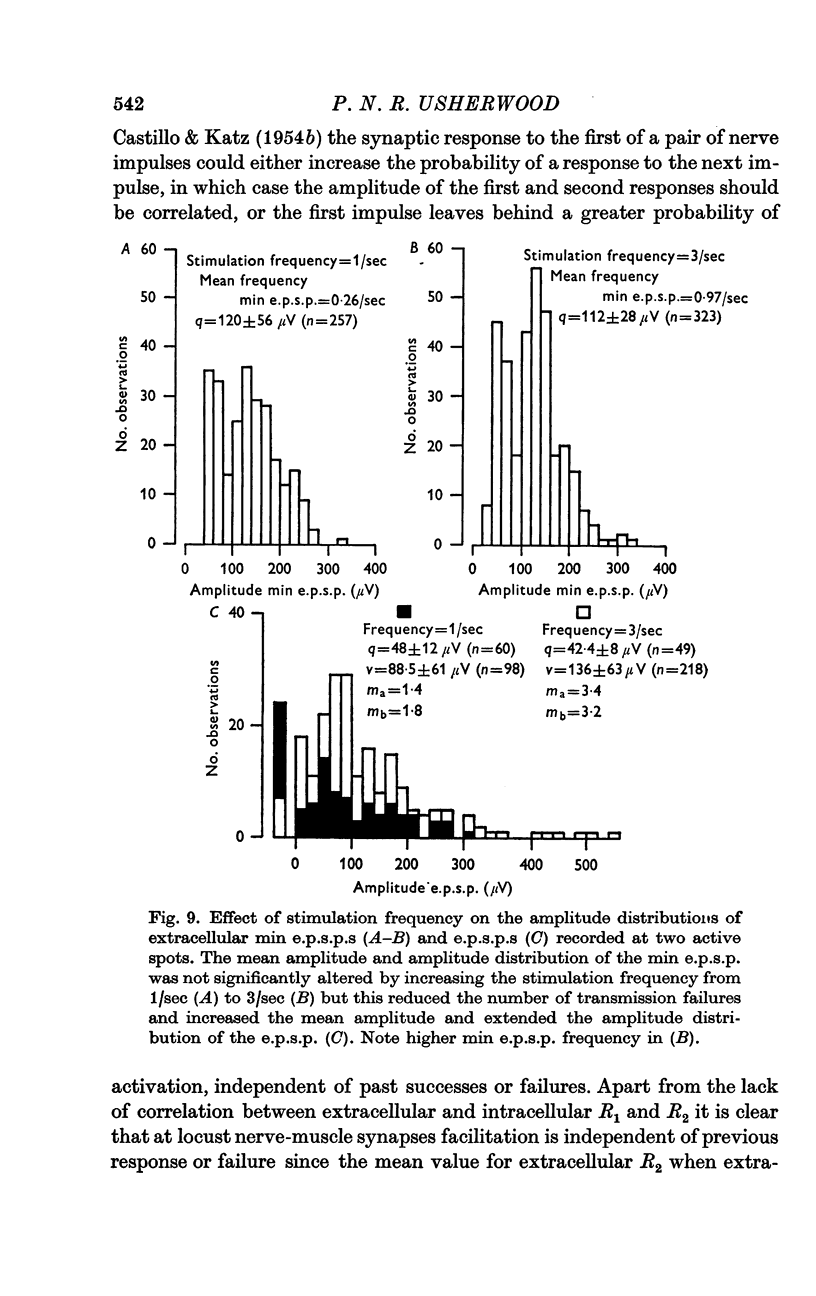
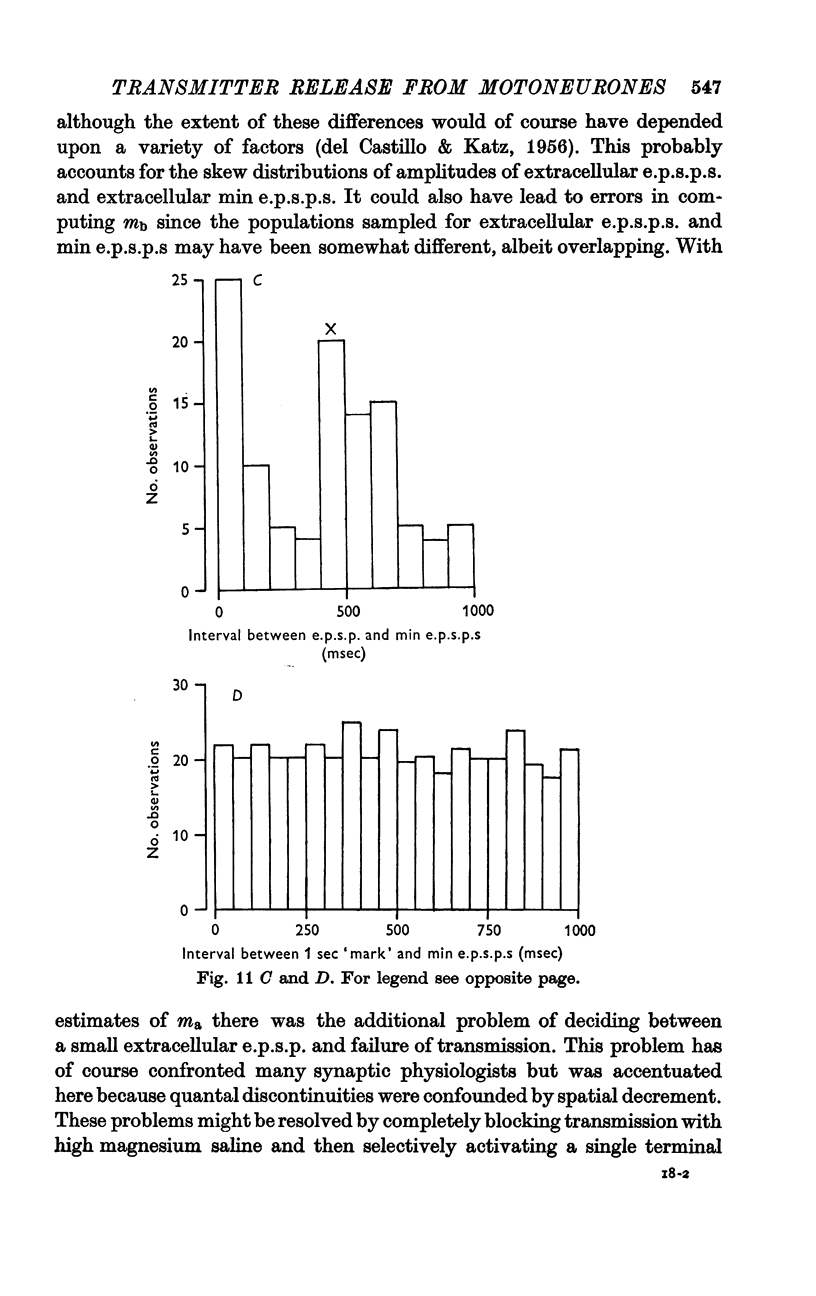
2. At most extracellular recording sites the amplitude distributions of the excitatory post-synaptic potentials (e.p.s.p.s) were apparently non-Poisson. However, interpretation of these amplitude distributions was complicated by the effect on the extracellular recordings of the complex structural organization of the retractor unguis nerve terminal with its spatially distinct transmitter release sites extending over distances of 15-30 μm.
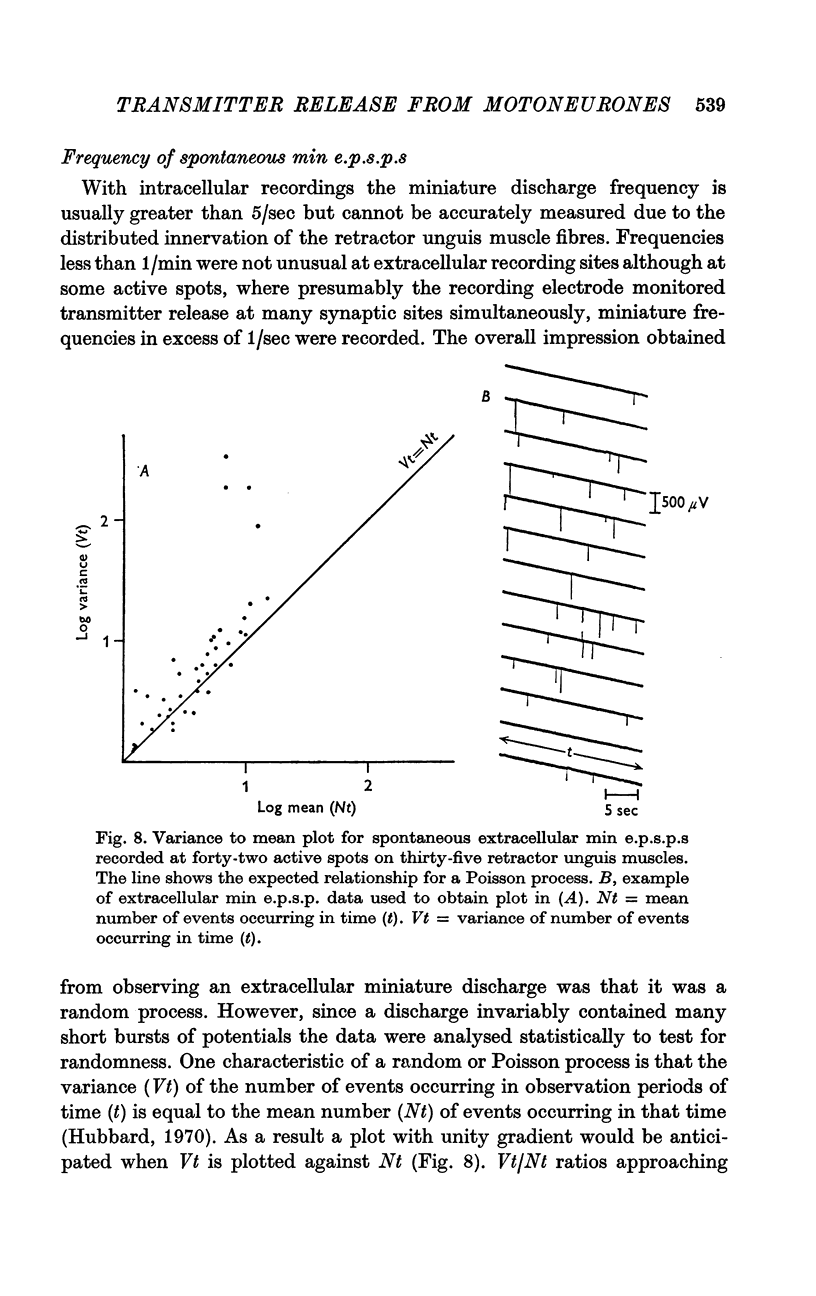
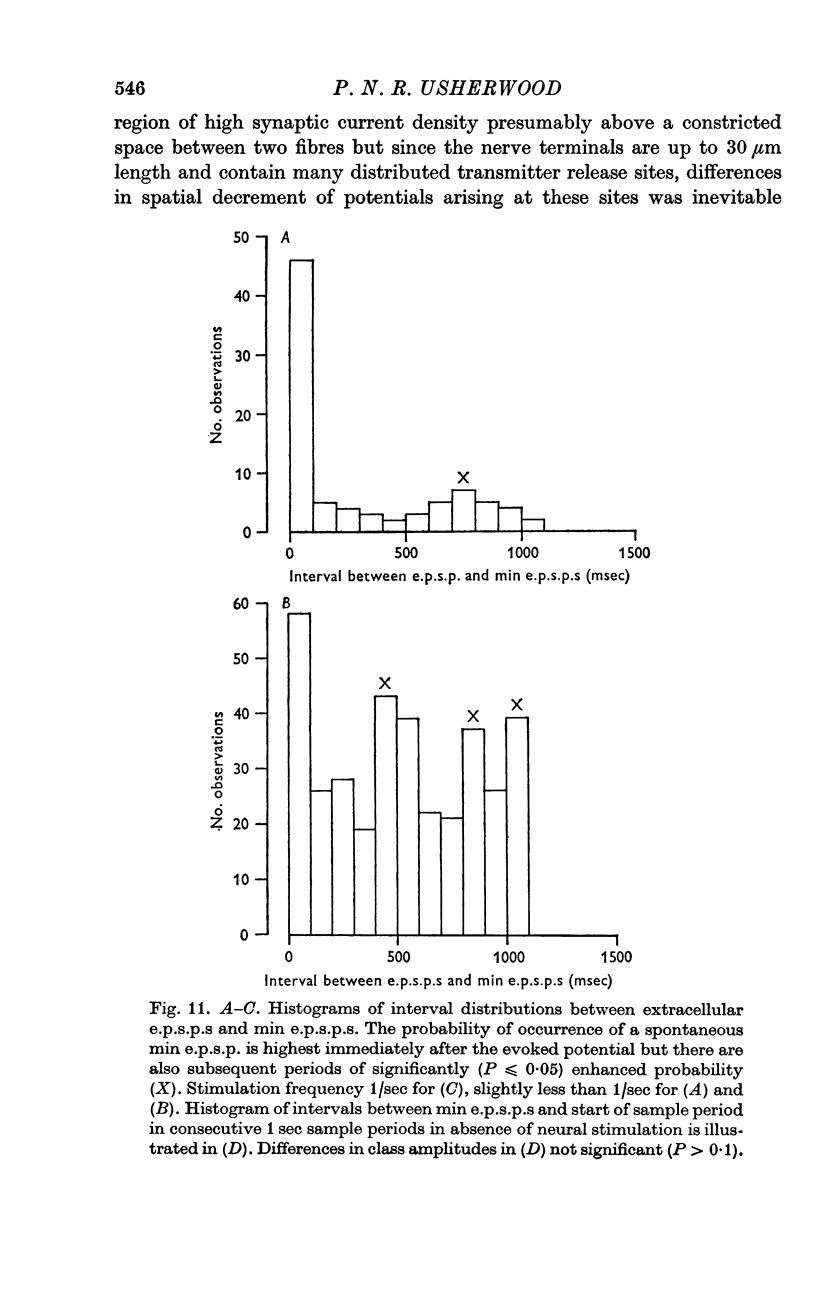
3. The spontaneous miniature excitatory post-synaptic potentials (min e.p.s.p.s) did not occur at random intervals, bursts of min e.p.s.p.s being frequently recorded. As a result the spontaneous release of transmitter rarely approximated a Poisson process.
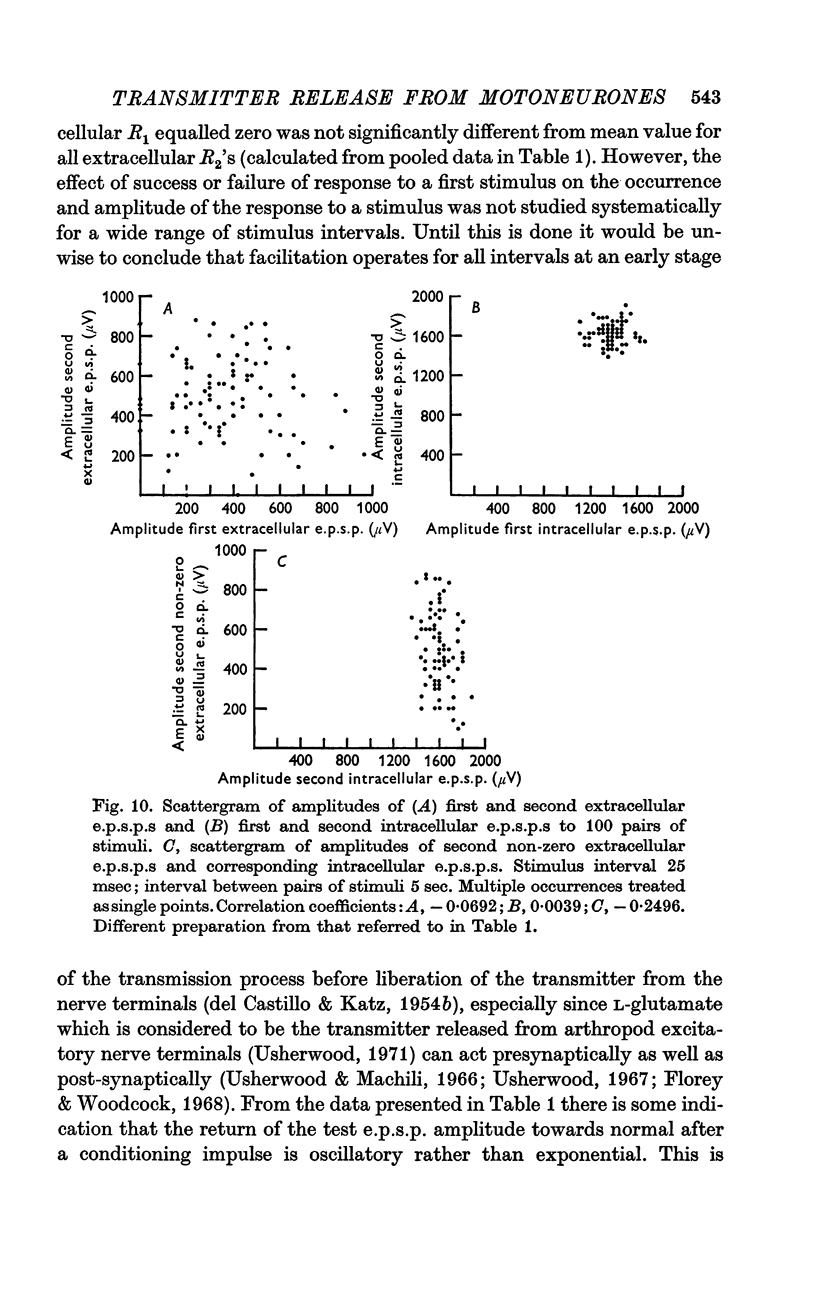
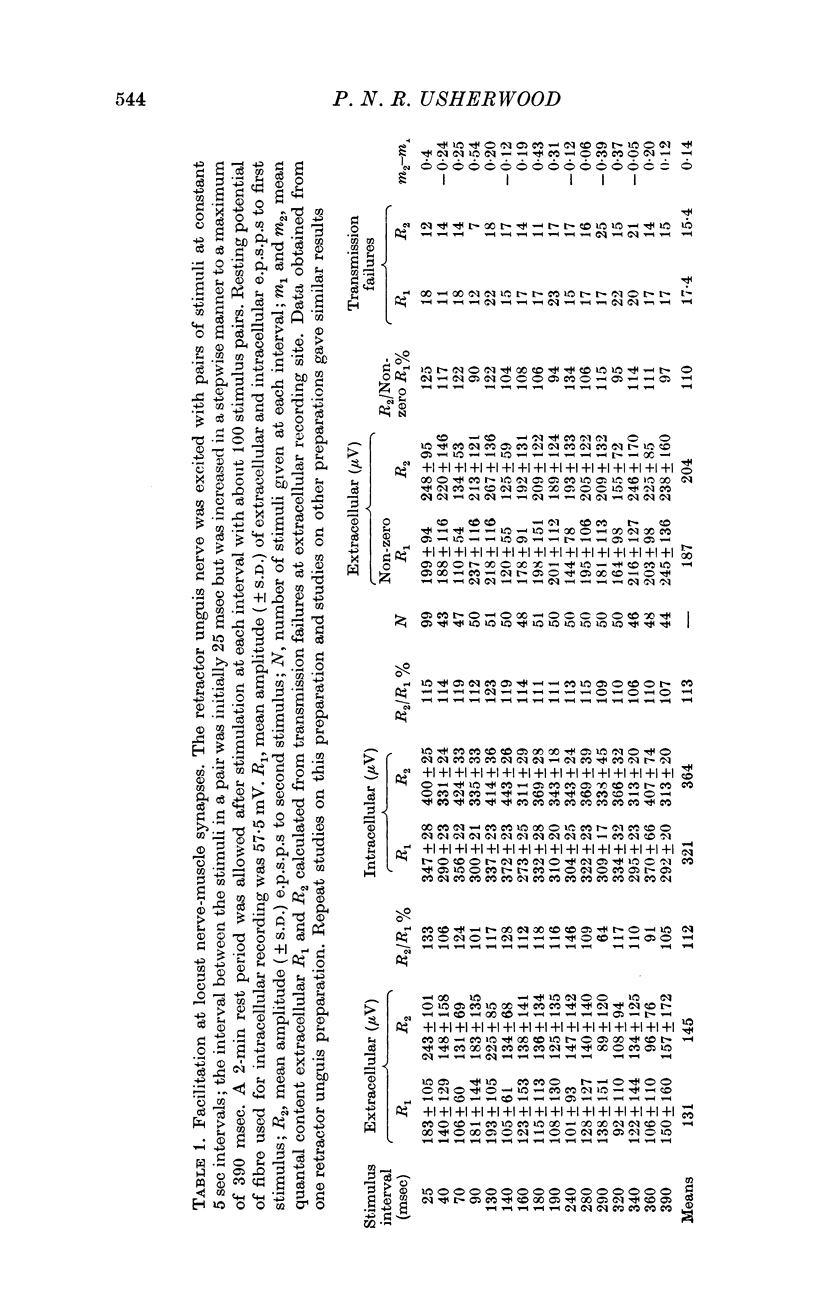
4. For a period of at least 390 msec following a conditioning nerve impulse a test e.p.s.p. was facilitated and the probability of spontaneous transmitter release was enhanced. A large primary phase of facilitation of impulse-linked and spontaneous release was invariably followed by one or more secondary phases of smaller magnitude.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D., Harrison J. A reconsideration of the Poisson hypothesis for transmitter release at the crayfish neuromuscular junction. J Physiol. 1970 Jan;206(1):1–23. doi: 10.1113/jphysiol.1970.sp008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Florey E., Woodcock B. Presynaptic excitatory action of glutamate applied to crab nerve-muscle preparations. Comp Biochem Physiol. 1968 Aug;26(2):651–661. doi: 10.1016/0010-406x(68)90657-9. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. Evidence for a Poisson distribution of miniature end-plate potentials and some implications. Nature. 1965 Oct 23;208(5008):395–396. doi: 10.1038/208395a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D., Usherwood P. N. Fine structure of normal and degenerating motor axons and nerve-muscle synapses in the locust, Schistocerca gregaria. Comp Biochem Physiol A Comp Physiol. 1972 Sep 1;43(1):83–101. doi: 10.1016/0300-9629(72)90471-9. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P. N. Spontaneous miniature potentials from insect muscle fibres. Nature. 1961 Aug 19;191:814–815. doi: 10.1038/191814a0. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N. Insect neuromuscular mechanisms. Am Zool. 1967 Aug;7(3):553–582. doi: 10.1093/icb/7.3.553. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N., Machili P. Chemical transmission at the insect excitatory neuromuscular synapse. Nature. 1966 May 7;210(5036):634–636. doi: 10.1038/210634a0. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N., Rees D. Quantitative studies of the spatial distribution of synaptic vesicles within normal and degenerating motor axons of the locust, Schistocerca gregaria. Comp Biochem Physiol A Comp Physiol. 1972 Sep 1;43(1):103–118. doi: 10.1016/0300-9629(72)90472-0. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N. Spontaneous miniature potentials from insect muscle fibres. J Physiol. 1963 Nov;169(1):149–160. doi: 10.1113/jphysiol.1963.sp007246. [DOI] [PMC free article] [PubMed] [Google Scholar]


