Abstract
1. The characteristics of renal accumulation of phenol red and p-aminohippurate (PAH) by slices of rabbit kidney cortex suspended in an electrolyte medium have been compared.
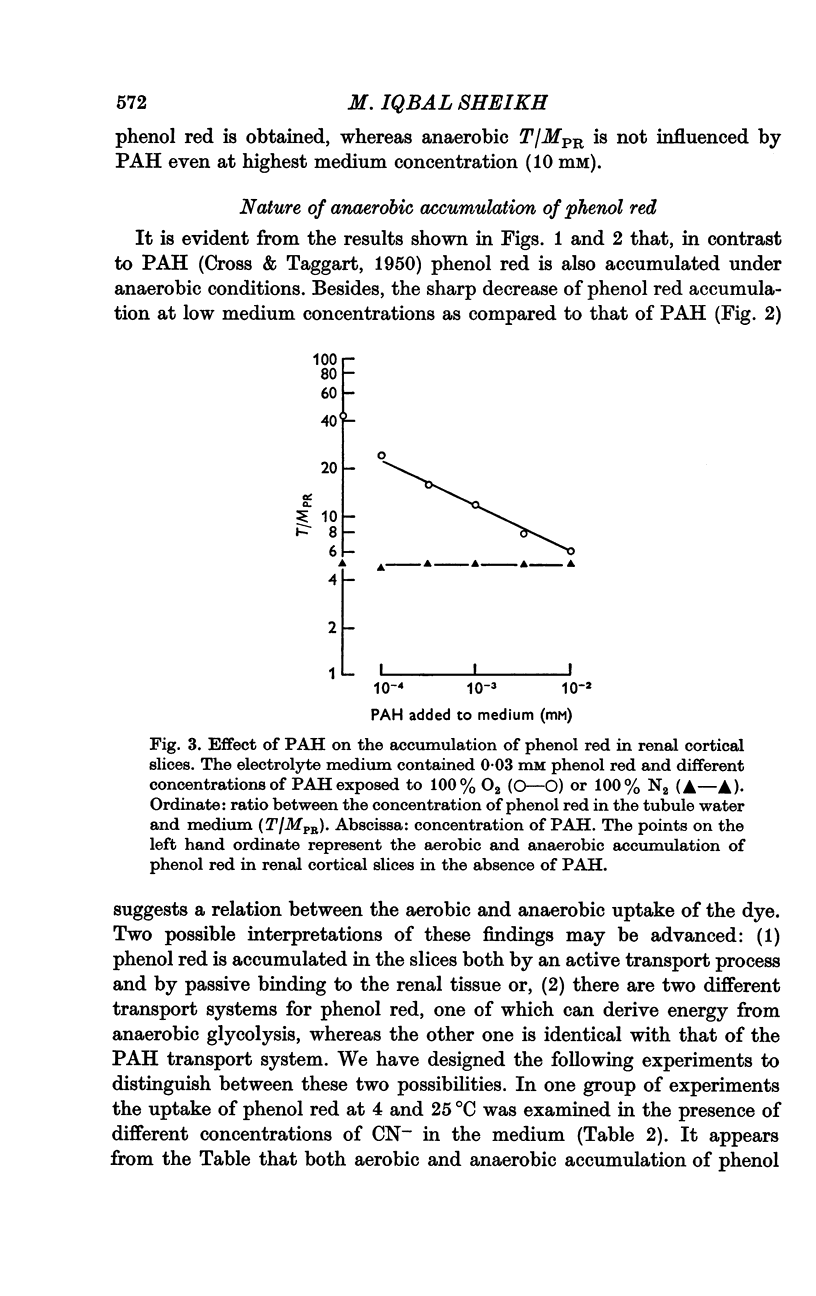
2. It has been found that at low medium concentrations the accumulation of phenol red is about 4-5 times as high as that of PAH. Furthermore, phenol red is accumulated by the renal tissue under anaerobic conditions, in contrast to PAH.
3. Experiments involving incubation of slices at low temperatures and addition of various metabolic inhibitors to the medium, indicate that the anaerobic accumulation of phenol red is due to binding to cellular constituents. This conclusion is corroborated by studies on renal homogenates from which it appears that phenol red is bound predominantly to the microsomal and mitochondrial fraction.
4. The aerobic accumulation of phenol red is less susceptible to inhibition by probenecid, 2,4-dinitrophenol (DNP), and octanoate than is that of PAH. Besides, probenecid, DNP, and octanoate inhibit phenol red binding to the microsomal fraction, whereas mitochondrial binding of phenol red is unaffected by the presence of these substances.
5. Fumarate and succinate affect the aerobic accumulation of phenol red and PAH to the same degree. Furthermore, fumarate, succinate, and PAH do not alter anaerobic accumulation of phenol red.
6. It is concluded that probenecid, DNP, and octanoate cause more inhibition of organic anion transport than fumarate, succinate, and PAH because of lipophilic interaction with the membrane. The pronounced resistance of phenol red accumulation to inhibition by lipophilic inhibitors is probably due to the ability of the indicator dye to displace these substances from binding sites on the transporting membranes.
Full text
PDF
























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEYER K. H., RUSSO H. F., TILLSON E. K., MILLER A. K., VERWEY W. F., GASS S. R. 'Benemid,' p-(di-n-propylsulfamyl)-benzoic acid; its renal affinity and its elimination. Am J Physiol. 1951 Sep;166(3):625–640. doi: 10.1152/ajplegacy.1951.166.3.625. [DOI] [PubMed] [Google Scholar]
- BURG M. B., ORLOFF J. Oxygen consumption and active transport in separated renal tubules. Am J Physiol. 1962 Aug;203:327–330. doi: 10.1152/ajplegacy.1962.203.2.327. [DOI] [PubMed] [Google Scholar]
- Barac-Nieto M., Cohen J. J. Nonesterified fatty acid uptake by dog kidney: effects of probenecid and chlorothiazide. Am J Physiol. 1968 Jul;215(1):98–107. doi: 10.1152/ajplegacy.1968.215.1.98. [DOI] [PubMed] [Google Scholar]
- Barác-Nieto M. Renal uptake of p-aminohippuric acid in vitro. Effects of palmitate and L-carnitine. Biochim Biophys Acta. 1971 Apr 13;233(2):446–452. doi: 10.1016/0005-2736(71)90341-5. [DOI] [PubMed] [Google Scholar]
- Berndt W. O., Grote D. The accumulation of C14-dinitrophenol by slices of rabbit kidney cortex. J Pharmacol Exp Ther. 1968 Nov;164(1):223–231. [PubMed] [Google Scholar]
- Berndt W. O. Probenecid binding by renal cortical slices and homogenates. Proc Soc Exp Biol Med. 1967 Oct;126(1):123–126. doi: 10.3181/00379727-126-32382. [DOI] [PubMed] [Google Scholar]
- Berndt W. O. Probenecid uptake by slices of rabbit kidney cortex. Biochem Pharmacol. 1966 Dec;15(12):1947–1956. doi: 10.1016/0006-2952(66)90223-1. [DOI] [PubMed] [Google Scholar]
- COPENHAVER J. H., Jr, FORSTER R. P. Intracellular accumulation as an active process in a mammalian renal transport system in vitro; energy dependence and competitive phenomena. Am J Physiol. 1956 Jul;186(1):167–171. doi: 10.1152/ajplegacy.1956.186.1.167. [DOI] [PubMed] [Google Scholar]
- CROSS R. J., TAGGART J. V. Renal tubular transport: accumulation of p-aminohippurate by rabbit kidney slices. Am J Physiol. 1950 Apr 1;161(1):181–190. doi: 10.1152/ajplegacy.1950.161.1.181. [DOI] [PubMed] [Google Scholar]
- Cortney M. A., Mylle M., Lassiter W. E., Gottschalk C. W. Renal tubular transport of water, solute, and PAH in rats loaded with isotonic saline. Am J Physiol. 1965 Dec;209(6):1199–1205. doi: 10.1152/ajplegacy.1965.209.6.1199. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deetjen P., Sonnenbeeg H. Der tubuläre Transport von p-Aminohippursäure. Mikroperfusionsversuche am Einzelnephron der Rattenniere in situ. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Jul 16;285(1):35–44. [PubMed] [Google Scholar]
- Despopoulos A. Congruence of renal and hepatic excretory functions: sulfonic acid dyes. Am J Physiol. 1971 Jun;220(6):1755–1758. doi: 10.1152/ajplegacy.1971.220.6.1755. [DOI] [PubMed] [Google Scholar]
- FORSTER R. P., HONG S. K. Tubular transport maxima of PAH and diodrast measured individually in the aglomerular kidney of Lophius, and simultaneously as competitors under conditions of equimolar loading. J Gen Physiol. 1962 Mar;45:811–820. doi: 10.1085/jgp.45.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes E. C. Kinetics of p-aminohippurate secretion in the rabbit. Am J Physiol. 1963 Nov;205(5):1019–1024. doi: 10.1152/ajplegacy.1963.205.5.1019. [DOI] [PubMed] [Google Scholar]
- HUANG K. C., LIN D. S. KINETIC STUDIES ON TRANSPORT OF PAH AND OTHER ORGANIC ACIDS IN ISOLATED RENAL TUBULES. Am J Physiol. 1965 Feb;208:391–396. doi: 10.1152/ajplegacy.1965.208.2.391. [DOI] [PubMed] [Google Scholar]
- Hong S. K., Park Y. S. Transport of bromcresol green in the rabbit kidney slice. Am J Physiol. 1971 Dec;221(6):1779–1784. doi: 10.1152/ajplegacy.1971.221.6.1779. [DOI] [PubMed] [Google Scholar]
- JOSEPHSON B., GRIEG A., KAKOSSAIOS G., KALLAS J. Renal tubular excretion from high plasma levels of para-aminohippurate (PAH) and diodrast (D) in unanaesthetized rabbits. Acta Physiol Scand. 1953 Dec 31;30(1):11–21. doi: 10.1111/j.1748-1716.1954.tb01070.x. [DOI] [PubMed] [Google Scholar]
- MALVIN R. L., WILDE W. S., SULLIVAN L. P. Localization of nephron transport by stop flow analysis. Am J Physiol. 1958 Jul;194(1):135–142. doi: 10.1152/ajplegacy.1958.194.1.135. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Regulation of cellular volume during anerobic incubation of rat renal cortical slices. Biochim Biophys Acta. 1968 Dec 10;163(4):557–559. doi: 10.1016/0005-2736(68)90085-0. [DOI] [PubMed] [Google Scholar]
- Maxild J., Moller J. V. Metabolic studies on renal transport of p-aminohippurate in vitro. Biochim Biophys Acta. 1969 Sep 2;184(3):614–624. doi: 10.1016/0304-4165(69)90276-1. [DOI] [PubMed] [Google Scholar]
- Maxild J. Role of fatty acid metabolism on renal transport of p-aminohippurate in vitro. Biochim Biophys Acta. 1971 Apr 13;233(2):434–445. doi: 10.1016/0005-2736(71)90340-3. [DOI] [PubMed] [Google Scholar]
- Moller J. V. The relation between secretion of urate and p-aminohippurate in the rabbit kidney. J Physiol. 1967 Sep;192(2):505–517. doi: 10.1113/jphysiol.1967.sp008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. S., Yoo H. S., Hong S. K. Kinetic studies on transport of organic acids in rabbit kidney slices. Am J Physiol. 1971 Jan;220(1):95–99. doi: 10.1152/ajplegacy.1971.220.1.95. [DOI] [PubMed] [Google Scholar]
- RESHEF L., SHAPIRO B. FATTY ACID ADSORPTION BY LIVER- AND ADIPOSE-TISSUE PARTICLES. Biochim Biophys Acta. 1965 Feb 1;98:73–80. doi: 10.1016/0005-2760(65)90012-3. [DOI] [PubMed] [Google Scholar]
- RODKEY F. L. Binding of phenol red by human serum albumin. Arch Biochem Biophys. 1961 Sep;94:526–531. doi: 10.1016/0003-9861(61)90081-9. [DOI] [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]
- SPERBER I. Competitive inhibition and specificity of renal tubular transport mechanisms. Arch Int Pharmacodyn Ther. 1954 Mar;97(2):221–231. [PubMed] [Google Scholar]
- Sheikh M. I., Moller J. V. The kinetic parameters of renal transport of p-aminohippurate in vitro. Biochim Biophys Acta. 1970;196(2):305–319. doi: 10.1016/0005-2736(70)90018-0. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Goldring W., Chasis H. THE MEASUREMENT OF THE TUBULAR EXCRETORY MASS, EFFECTIVE BLOOD FLOW AND FILTRATION RATE IN THE NORMAL HUMAN KIDNEY. J Clin Invest. 1938 May;17(3):263–278. doi: 10.1172/JCI100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetten M. R., Burnett F. F. Some properties of variously activated microsomal glucose-6-phosphatase, inorganic pyrophosphatase and inorganic pyrophosphate-glucose phosphotransferase. Shift in pH optimum. Biochim Biophys Acta. 1967 May 16;139(1):138–147. doi: 10.1016/0005-2744(67)90120-9. [DOI] [PubMed] [Google Scholar]
- TILLSON E. K., SCHUCHARDT G. S., FISHMAN J. K., BEYER K. H. The determination of probenecid (benemid) in body fluids. J Pharmacol Exp Ther. 1954 Aug;111(4):385–394. [PubMed] [Google Scholar]
- Tune B. M., Burg M. B., Patlak C. S. Characteristics of p-aminohippurate transport in proximal renal tubules. Am J Physiol. 1969 Oct;217(4):1057–1063. doi: 10.1152/ajplegacy.1969.217.4.1057. [DOI] [PubMed] [Google Scholar]
- WEINER I. M., WASHINGTON J. A., 2nd, MUDGE G. H. On the mechanism of action of probenecid on renal tubular secretion. Bull Johns Hopkins Hosp. 1960 Jun;106:333–346. [PubMed] [Google Scholar]
- Wedeen R. P., Thier S. O. Intrarenal distribution of nonmetabolized amino acids in vivo. Am J Physiol. 1971 Feb;220(2):507–512. doi: 10.1152/ajplegacy.1971.220.2.507. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]


