Abstract
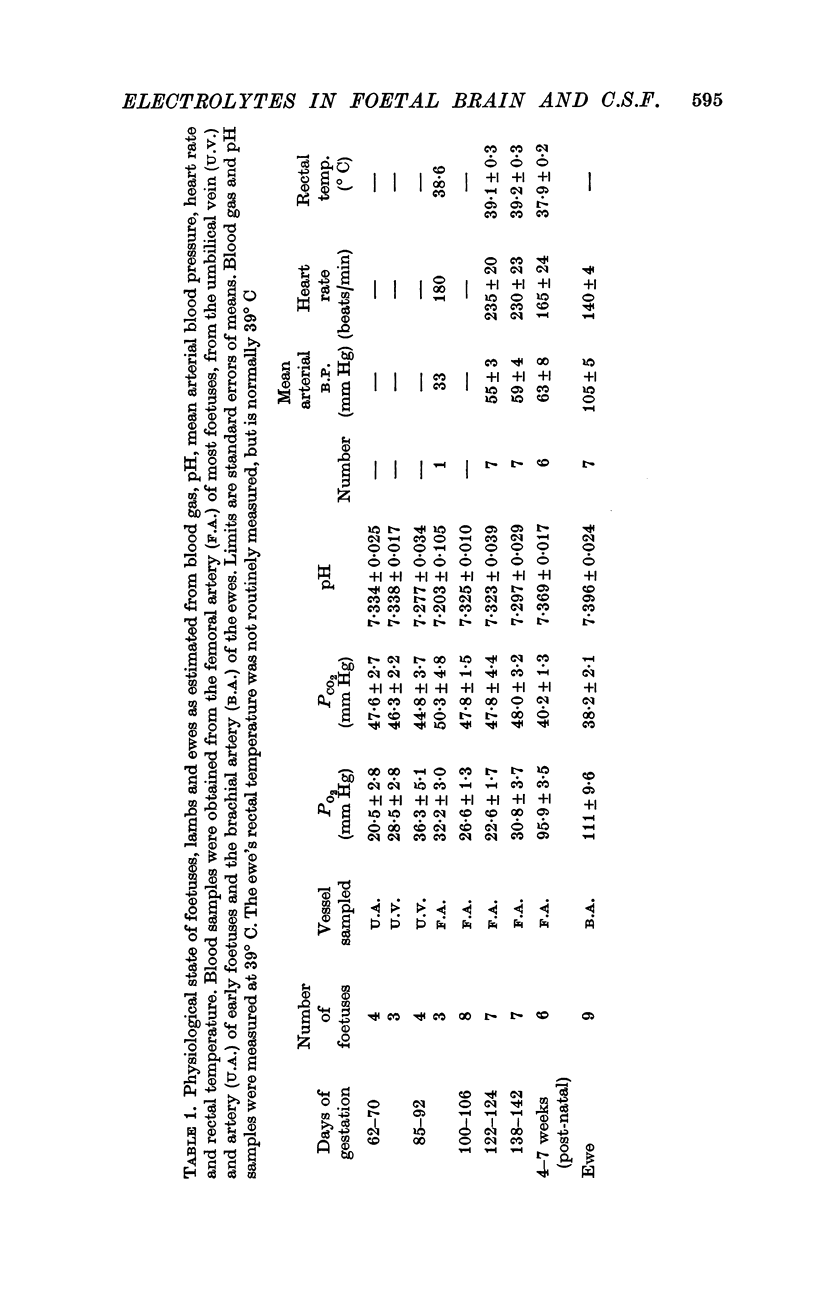
1. Samples of cisternal cerebrospinal fluid (c.s.f.) and blood plasma have been obtained nearly simultaneously from foetal sheep of different ages, the foetus having been exteriorized and maintained in a normal state with respect to its blood gases and arterial pH. The brains were removed from these foetuses and also from foetal guinea-pigs after exsanguination.
2. A comprehensive study has been made of the concentrations of water, chloride, sodium, potassium, calcium and magnesium in blood plasma, c.s.f. and brain from foetal sheep and in brain from foetal guinea-pigs during development in utero. Maternal arterial blood plasma, cisternal c.s.f. and brain from the sheep and brain from the guinea-pig have been analysed for comparison.
3. Concentrations of the ions analysed in foetal blood plasma are similar to those found by others in the sheep and other species. In the case of calcium, the results suggest an active maintenance of the concentration of this ion in foetal plasma by the placenta.
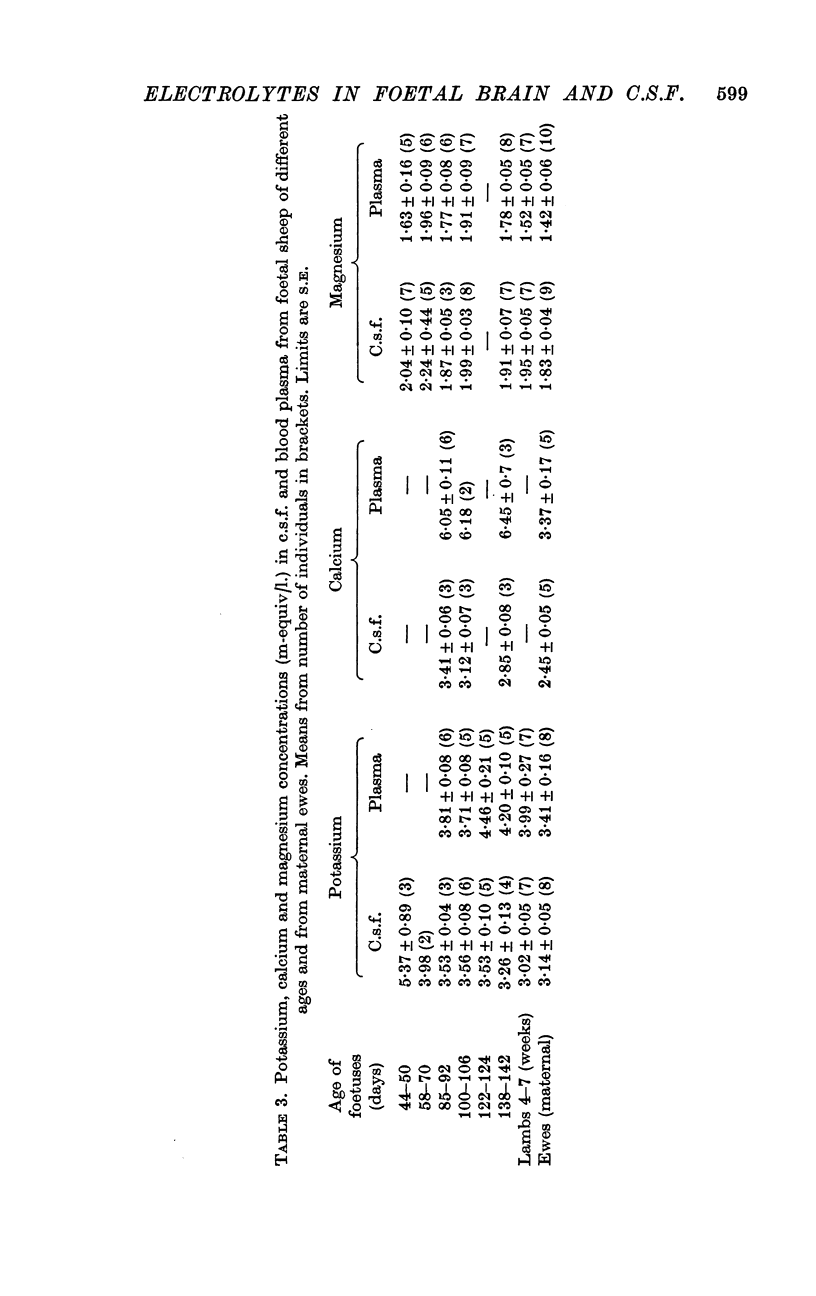
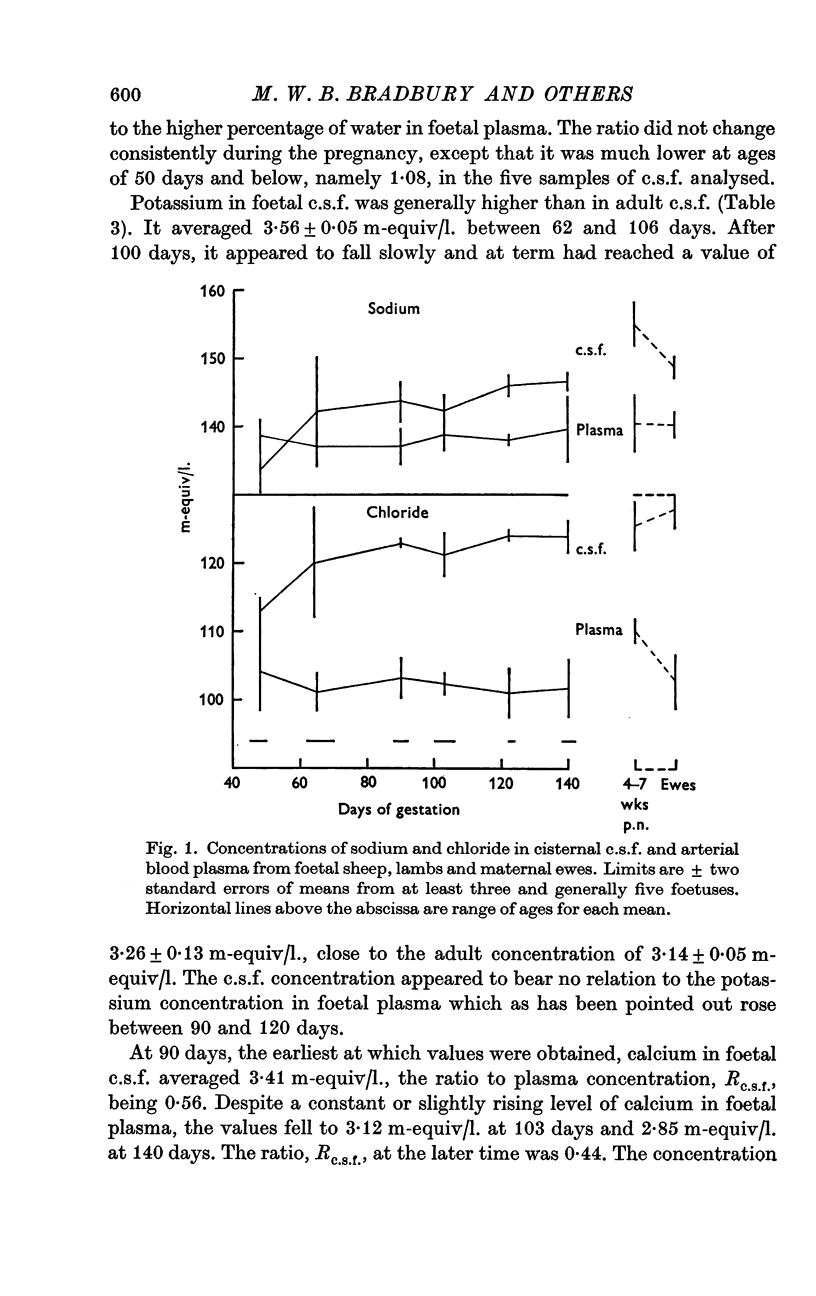
4. The chloride concentration in c.s.f. at ages between 65 days and term (147 days) averaged 1·19 times that in foetal plasma, but only 1·08 at 45-50 days; the sodium concentration in c.s.f. was also slightly reduced at this time. The increase in the c.s.f./plasma ratios for chloride and sodium appears to coincide with the first development of the blood—brain and blood—c.s.f. barriers to non-electrolytes.
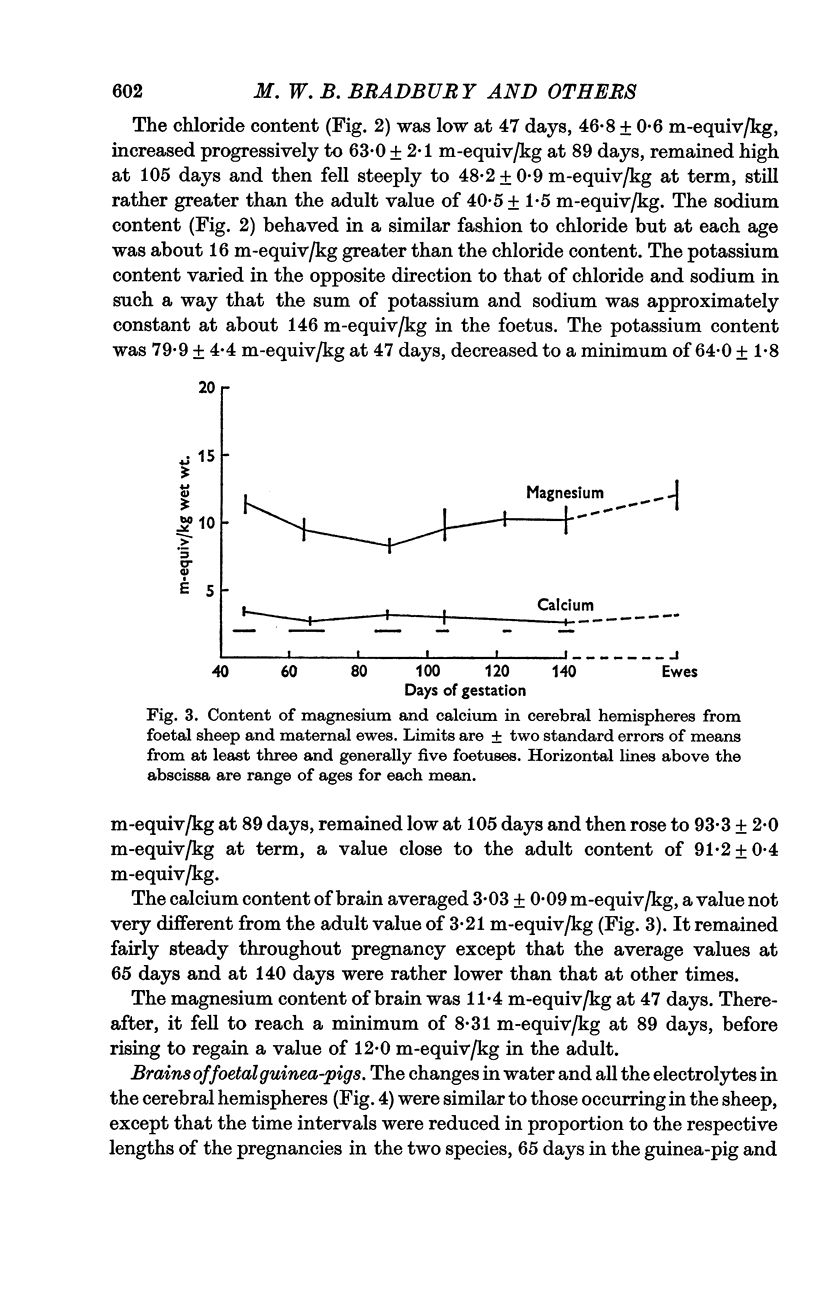
5. Magnesium was at a slightly higher concentration in c.s.f. than in plasma at all foetal ages and did not vary with age. The concentrations of potassium and calcium in c.s.f. were high at early ages and fell to reach adult concentrations after birth: the mechanisms determining the concentrations of the various ions in c.s.f. develop at very different, largely independent, rates.
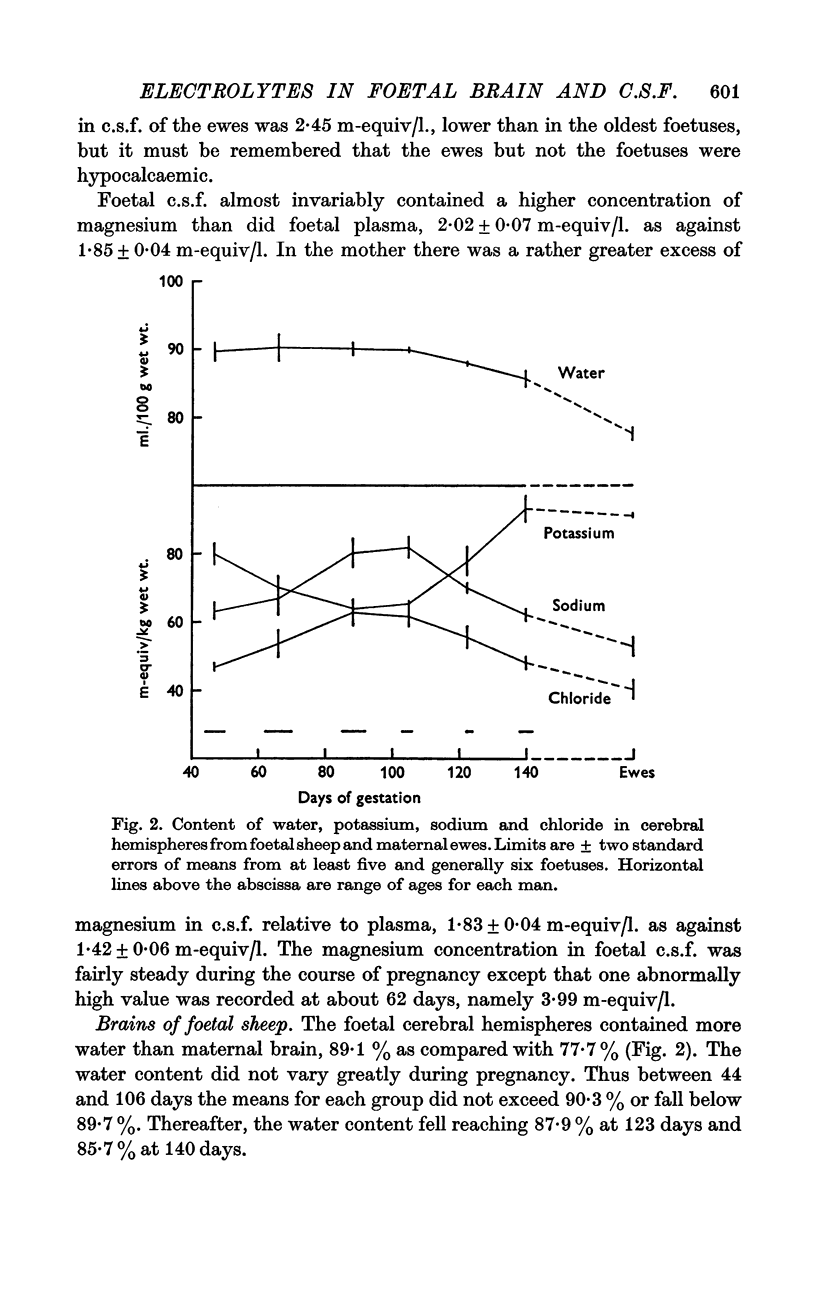
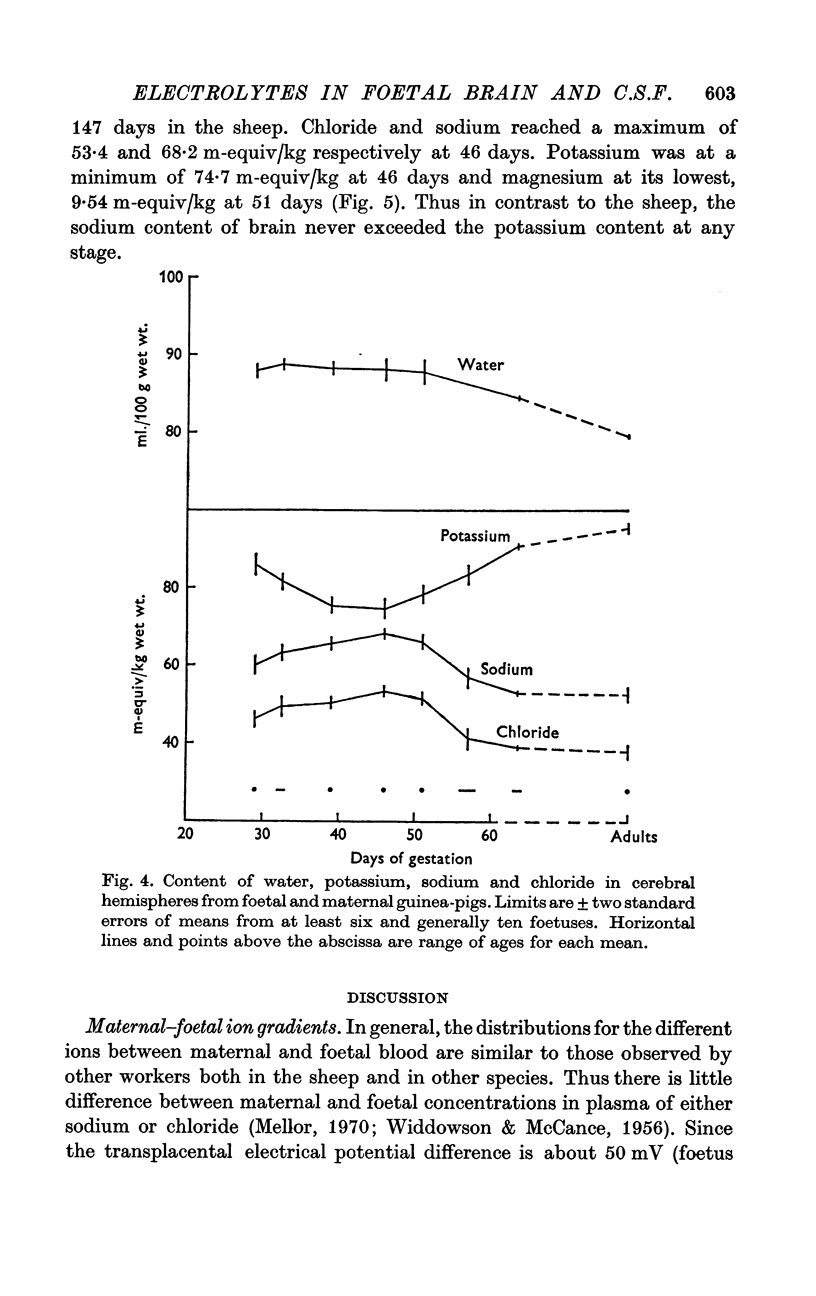
6. The water content of the cerebral hemispheres of the foetal sheep was stable at 90% of the wet weight till 105 days and fell thereafter. The contents of chloride, sodium and potassium followed paraboloid relations with age. Chloride and sodium reached a peak of 62 and 81 m-equiv/kg respectively between 89 and 105 days in the sheep. Potassium was at a minimum of 65 m-equiv/kg at the same time. The content of water and these electrolytes in the cerebral hemispheres of the foetal guinea-pig underwent similar changes, the maxima and minimum occurring as in the sheep at two-thirds of the total length of the pregnancy, namely 65 days in the guinea-pig. At 46 days in the guinea-pig, chloride in brain reached 53 m-equiv/kg, sodium was 68 m-equiv/kg and potassium was 75 m-equiv/kg. In contrast to the sheep, no reversal of the sodium-potassium ratio occurred. These changes in water and electrolytes probably represent a rise, a peak and a decrease in the volume of the extracellular space of cerebral cortex, but changes in the volume occupied by a cell-type, containing much intracellular chloride and sodium, could also contribute to this phenomenon.
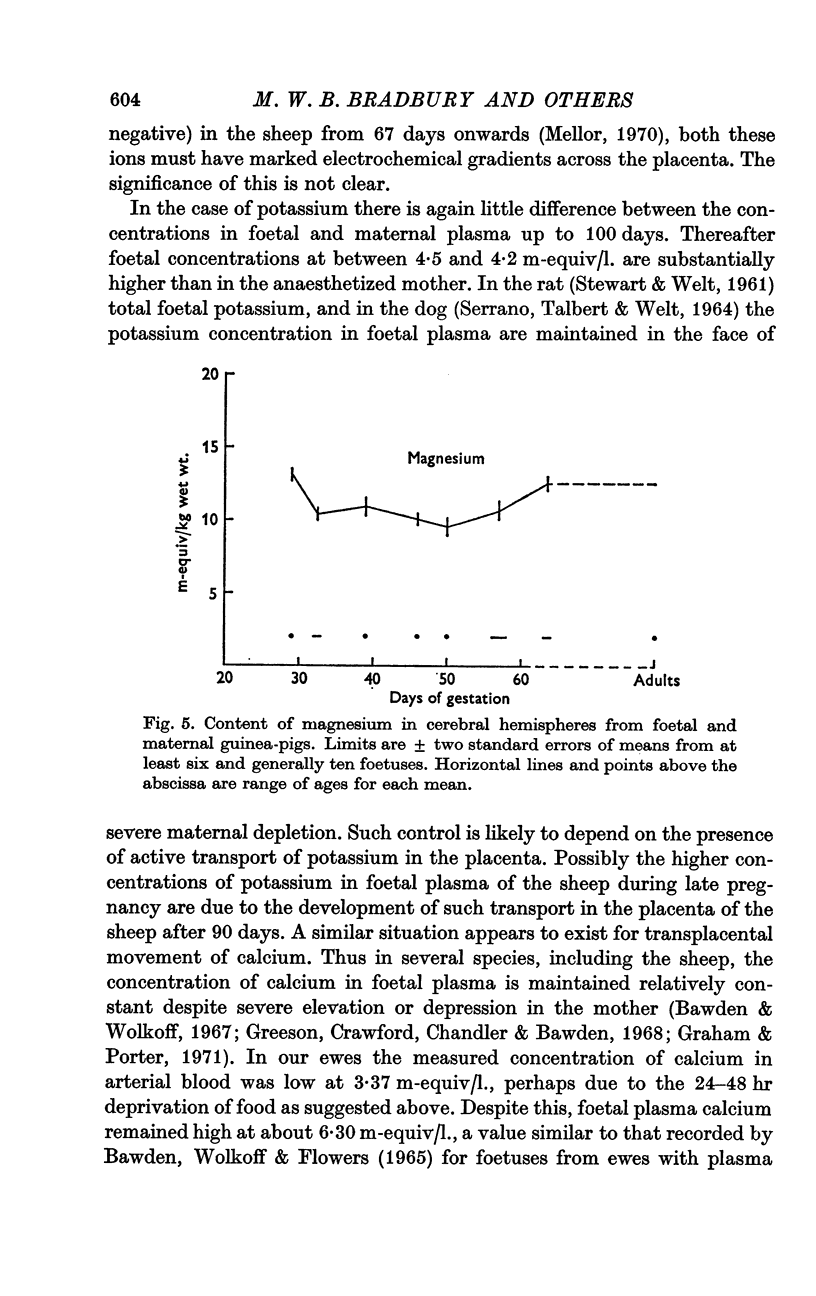
7. The calcium content in the cerebral hemispheres of the foetal sheep remained at about 3·0 m-equiv/kg throughout pregnancy. Magnesium in the cerebral hemispheres of both the foetal sheep and the guinea-pig showed a trough in concentration during pregnancy which corresponded approximately in position to the minimum in potassium content and the maxima in chloride and sodium contents. Lowest values were 9·6 m-equiv/kg at 105 days in the sheep and 9·5 m-equiv/kg at 51 days in the guinea-pig.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aström K. E. On the early development of the isocortex in fetal sheep. Prog Brain Res. 1967;26:1–59. [PubMed] [Google Scholar]
- BAWDEN J. W., WOLKOFF A. S., FLOWERS C. E. MATERNAL-FETAL BLOOD CALCIUM RELATIONSHIPS IN SHEEP. Obstet Gynecol. 1965 Apr;25:548–552. [PubMed] [Google Scholar]
- Bawden J. W., Wolkoff A. S. Fetal blood calcium responses to maternal calcium infusion in sheep. Am J Obstet Gynecol. 1967 Sep 1;99(1):55–60. doi: 10.1016/s0002-9378(16)34490-8. [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Myers R. E. The ontogenesis of haematoencephalic cation transport processes in the rhesus monkey. J Physiol. 1970 May;208(1):153–170. doi: 10.1113/jphysiol.1970.sp009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W., Pysh J. J. Distribution of the extracellular space during postnatal maturation of rat cerebral cortex. Anat Rec. 1968 Apr;160(4):773–780. doi: 10.1002/ar.1091600412. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Leeman C. R., Bagdoyanh, Berberian A. The calcium and magnesium content of skeletal muscle, brain, and cerebrospinal fluid as determined by atomic bsorption flame photometry. J Lab Clin Med. 1968 May;71(5):884–892. [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., WIDDICOMBE J. G., WYATT D. G. Changes in the lungs of the new-born lamb. J Physiol. 1953 Jul;121(1):141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza S. W., Dobbing J. Cerebral edema in developing brain. I. Normal water and cation content in developing rat brain and postmortem changes. Exp Neurol. 1971 Sep;32(3):431–438. doi: 10.1016/0014-4886(71)90009-4. [DOI] [PubMed] [Google Scholar]
- FLEXNER L. B., FLEXNER J. B. Biochemical and physiological differentiation during morphogenesis; the extracellular and intracellular phases of the liver and cerebral cortex of the fetal guinea pig as estimated from distribution of chloride and radiosodium. J Cell Physiol. 1949 Aug;34(1):115–127. doi: 10.1002/jcp.1030340108. [DOI] [PubMed] [Google Scholar]
- Ferguson R. K., Woodbury D. M. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp Brain Res. 1969;7(3):181–194. doi: 10.1007/BF00239028. [DOI] [PubMed] [Google Scholar]
- Graham R. W., Porter G. P. Fetal-maternal plasma calcium relationships in the rabbit. Q J Exp Physiol Cogn Med Sci. 1971 Jul;56(3):160–168. doi: 10.1113/expphysiol.1971.sp002115. [DOI] [PubMed] [Google Scholar]
- Greeson C. D., Crawford E. G., Jr, Chandler D. C., Jr, Bawden J. W. Fetal blood calcium response to maternal hypercalcemia in guinea pigs. J Dent Res. 1968 May-Jun;47(3):447–449. doi: 10.1177/00220345680470031701. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- MCCANCE R. A., WIDDOWSON E. M. The effect of development on the composition of the serum and extracellular fluids. Clin Sci. 1956 Aug;15(3):361–365. [PubMed] [Google Scholar]
- Mellor D. J. Distribution of ions and electrical potential differences between mother and foetus at different gestational ages in goats and sheep. J Physiol. 1970 Mar;207(1):133–150. doi: 10.1113/jphysiol.1970.sp009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil E., O'Regan R. G. The effects of electrical stimulation of the distal end of the cut sinus and aortic nerves on peripheral arterial chemoreceptor activity in the cat. J Physiol. 1971 May;215(1):15–32. doi: 10.1113/jphysiol.1971.sp009455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRANO C. V., TALBERT L. M., WELT L. G. POTASSIUM DEFICIENCY IN THE PREGNANT DOG. J Clin Invest. 1964 Jan;43:27–31. doi: 10.1172/JCI104890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNADAKIS A., WOODBURY D. M. CELLULAR AND EXTRACELLULAR SPACES IN DEVELOPING RAT BRAIN. RADIOACTIVE UPTAKE STUDIES WITH CHLORIDE AND INULIN. Arch Neurol. 1965 Mar;12:284–293. doi: 10.1001/archneur.1965.00460270060008. [DOI] [PubMed] [Google Scholar]
- VERNADAKIS A., WOODBURY D. M. Electrolyte and amino acid changes in rat brain during maturation. Am J Physiol. 1962 Oct;203:748–752. doi: 10.1152/ajplegacy.1962.203.4.748. [DOI] [PubMed] [Google Scholar]
- Villegas J., Villegas L., Villegas R. Sodium, potassium, and chloride concentrations in the Schwann cell and axon of the squid nerve fiber. J Gen Physiol. 1965 Sep;49(1):1–7. doi: 10.1085/jgp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


