Abstract
1. The organization of receptive fields of retinal ganglion cells and A-laminae cells from the dorsal lateral geniculate nucleus (LGN) of the cat are compared under identical conditions. Some aspects of the geniculate data have been given elsewhere (Hammond, 1972b).
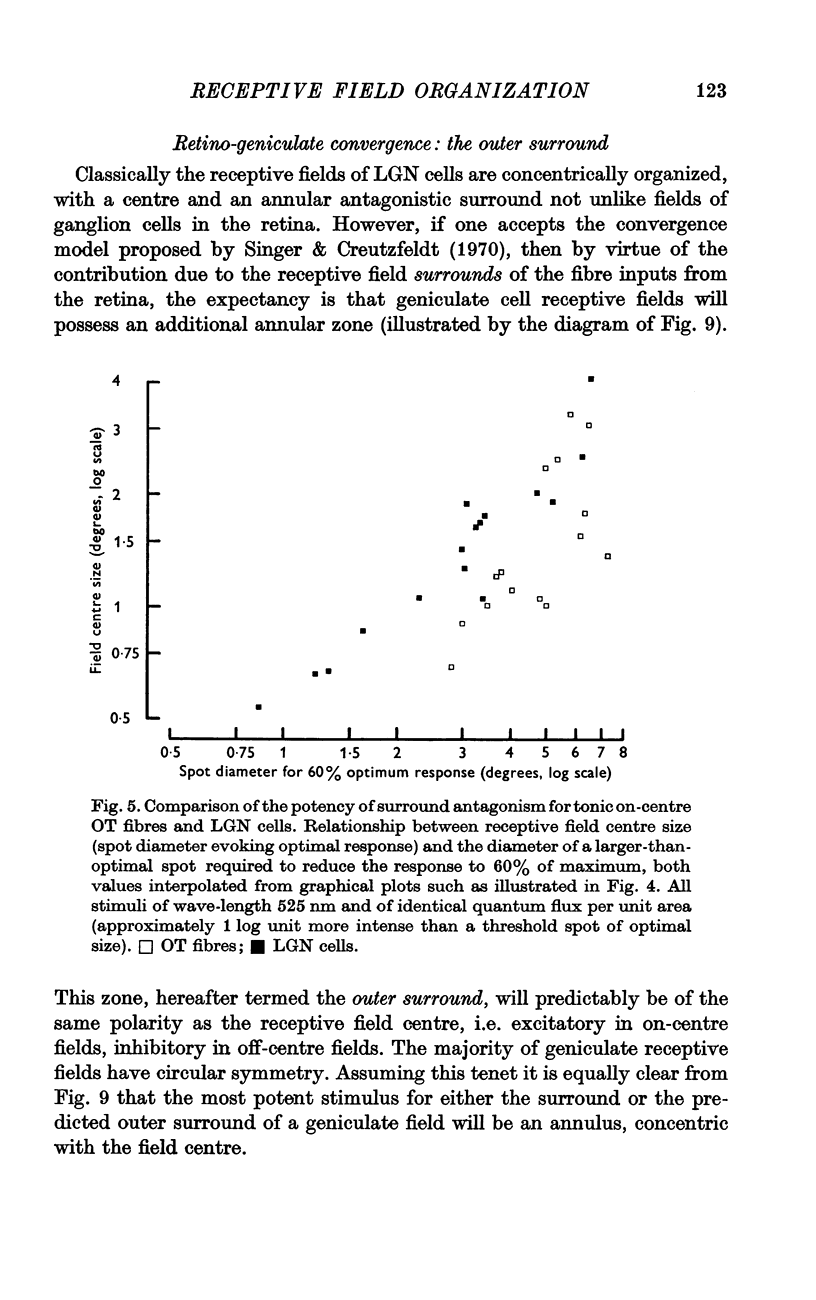
2. The receptive fields of geniculate cells consist of three zones — centre, antagonistic surround and synergistic outer surround — compared with only two for retinal cells. This result further supports the theory that the centre and surround of geniculate cell receptive fields derive from convergent, but discrete, retinal inputs.
3. The surrounds of geniculate receptive fields are known to be more powerfully antagonistic on their centres than is true of retinal cells. This relationship is re-examined.
4. Unlike geniculate fields, the locus of maximum sensitivity for the receptive field surround of retinal cells is not invariant either to stimulus geometry or adaptational state.
5. The latter result strongly suggests that the surround mechanism for retinal cells extends through the centre of the field. It establishes unequivocally that the overlap between receptive field centre and surround mechanisms, only marginal in geniculate, is very extensive indeed in retina.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971 Jun 9;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Saito H. Phasic and tonic cells in the cat's lateral geniculate nucleus. Tohoku J Exp Med. 1972 Feb;106(2):209–210. doi: 10.1620/tjem.106.209. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Saito H. The relationship between response characteristics to flicker stimulation and receptive field organization in the cat's optic nerve fibers. Vision Res. 1971 Mar;11(3):227–240. doi: 10.1016/0042-6989(71)90187-8. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Chromatic sensitivity and spatial organization of LGN neurone receptive fields in cat: cone-rod interaction. J Physiol. 1972 Sep;225(2):391–413. doi: 10.1113/jphysiol.1972.sp009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Chromatic sensitivity and spatial organization of cat visual cortical cells: cone-rod interaction. J Physiol. 1971 Mar;213(2):475–494. doi: 10.1113/jphysiol.1971.sp009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., James C. R. The Purkinje shift in cat: extent of the mesopic range. J Physiol. 1971 Jul;216(1):99–109. doi: 10.1113/jphysiol.1971.sp009511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Spatial organization of receptive fields of LGN neurones. J Physiol. 1972 Apr;222(1):53P–54P. [PubMed] [Google Scholar]
- Hammond P. Spectral properties of dark-adapted retinal ganglion cells in the plaice (Pleuronectes platessa, L.). J Physiol. 1968 Apr;195(3):535–556. doi: 10.1113/jphysiol.1968.sp008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Retinogeniculate convergence and analysis of contrast. J Neurophysiol. 1972 Jan;35(1):65–72. doi: 10.1152/jn.1972.35.1.65. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. I: contrast-sensitive units. J Neurophysiol. 1968 Mar;31(2):249–256. doi: 10.1152/jn.1968.31.2.249. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Spekreijse H., Wagner H. G., Wolbarsht M. L. Spectral and spatial coding of ganglion cell responses in goldfish retina. J Neurophysiol. 1972 Jan;35(1):73–86. doi: 10.1152/jn.1972.35.1.73. [DOI] [PubMed] [Google Scholar]
- Stone J., Freeman R. B., Jr Conduction velocity groups in the cat's optic nerve classified according to their retinal origin. Exp Brain Res. 1971 Nov 30;13(5):489–497. doi: 10.1007/BF00234279. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffman K. P. Conduction velocity as a parameter in the organisation of the afferent relay in the cat's lateral geniculate nucleus. Brain Res. 1971 Sep 24;32(2):454–459. doi: 10.1016/0006-8993(71)90339-8. [DOI] [PubMed] [Google Scholar]
- Stone J., Holländer H. Optic nerve axon diameters measured in the cat retina: some functional considerations. Exp Brain Res. 1971 Nov 30;13(5):498–503. doi: 10.1007/BF00234280. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]



