Abstract
1. In cats anaesthetized with Nembutal climbing fibre (CF) responses evoked in individual cerebellar Purkyně cells by mechanical stimulation of the skin were conditioned by preceding stimuli both to the periphery and to the precruciate area of the cerebral cortex.
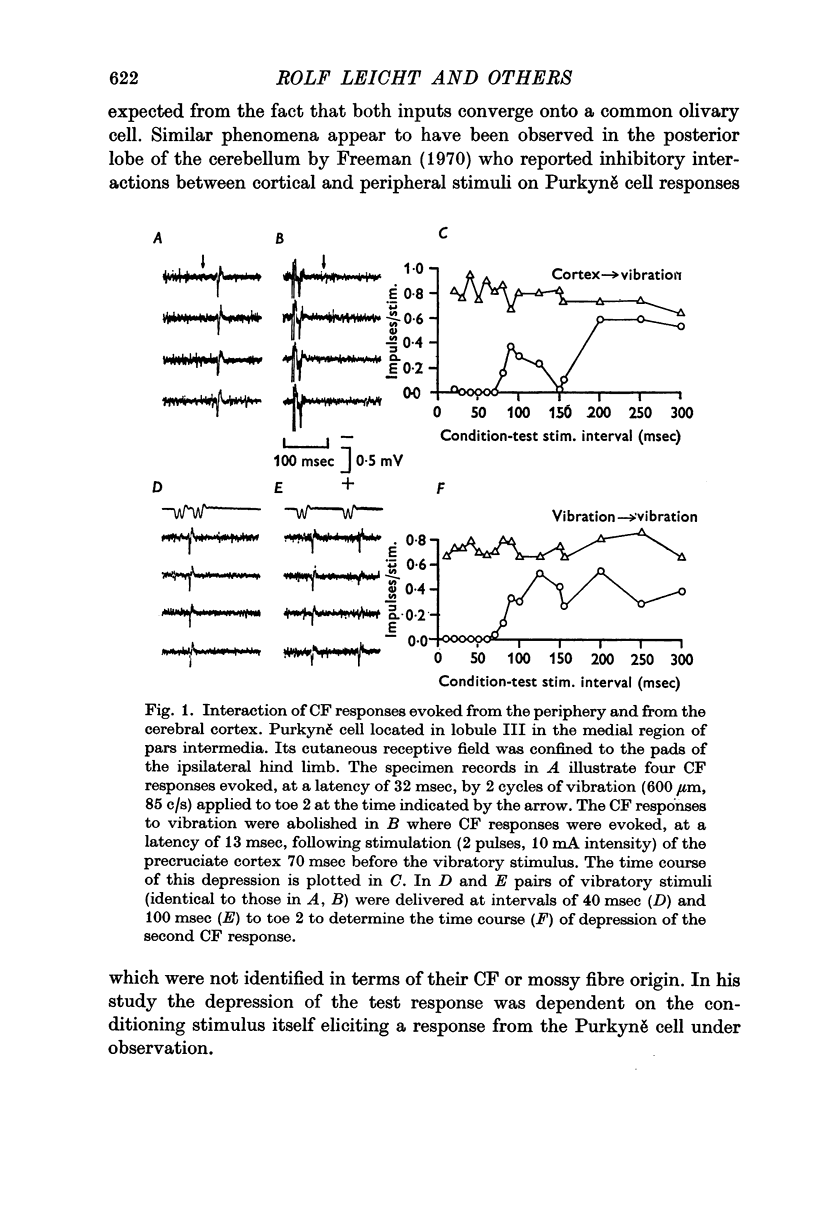
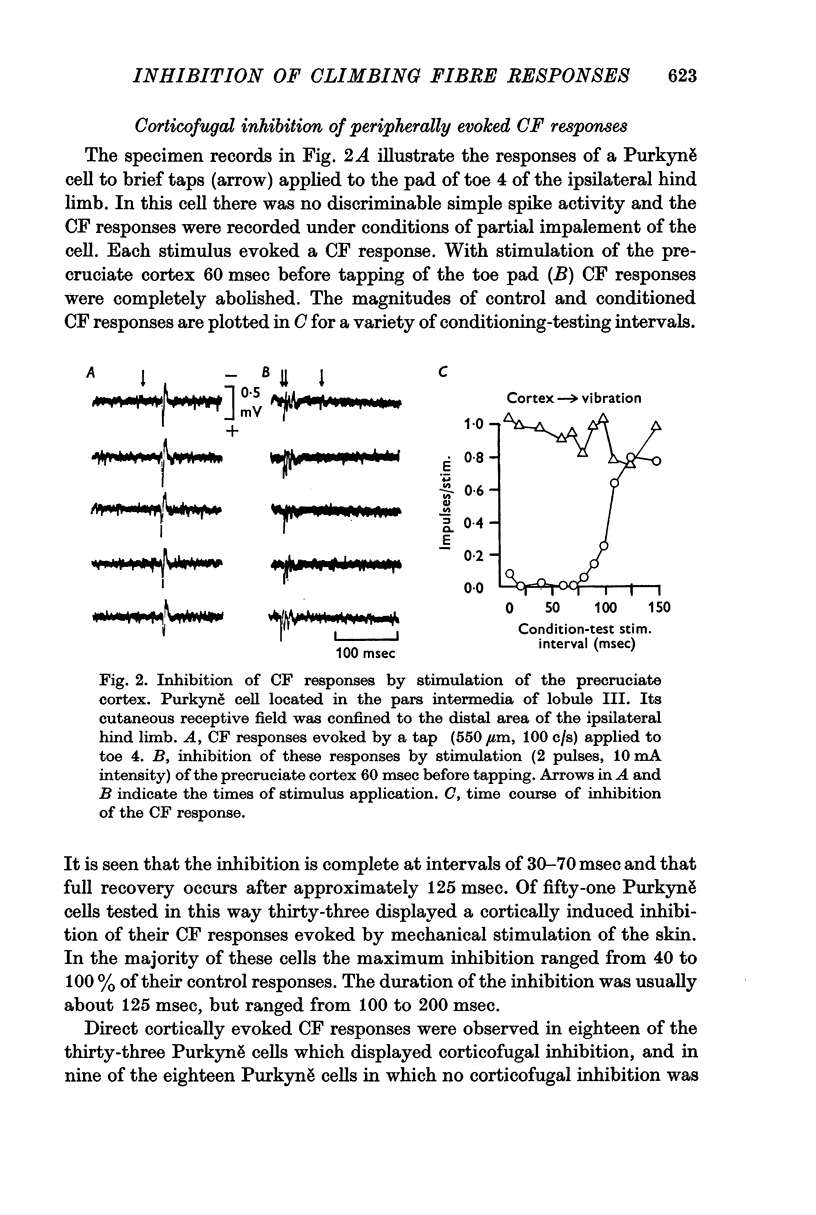
2. Cortical stimulation, generally subthreshold for evoking a CF response itself, induced an inhibition of cutaneous evoked CF responses in 65% of all Purkyně cells tested. The maximum inhibition ranged from 40 to 100% of the control responses at conditioning-testing intervals of 30-70 msec and the duration of the inhibition was usually 125 msec.
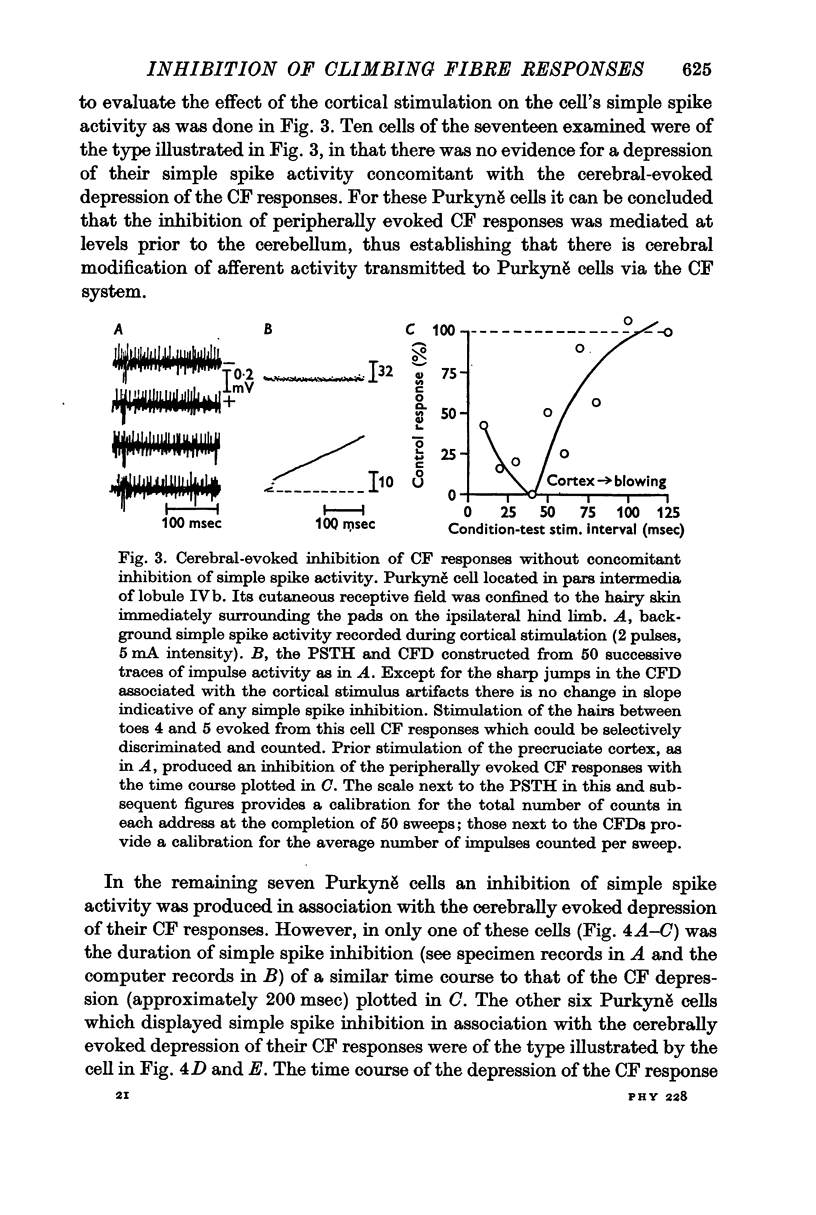
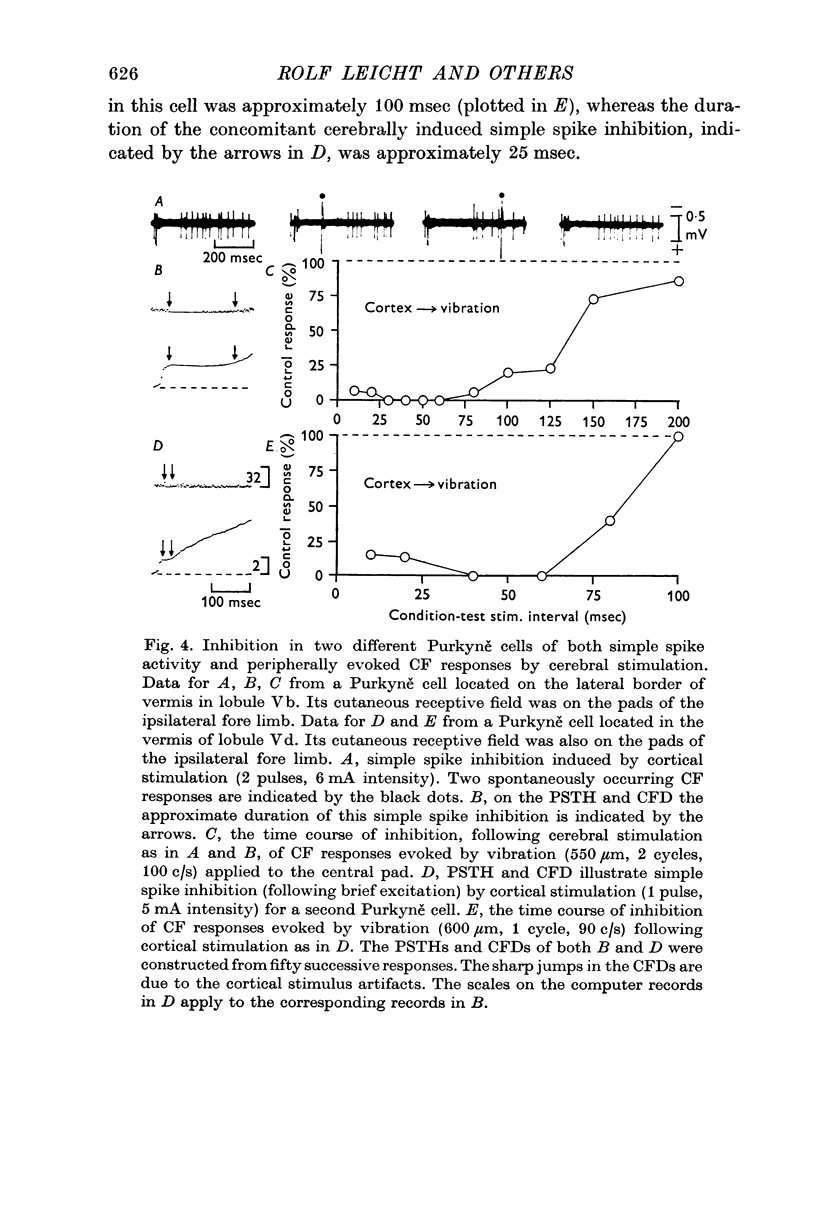
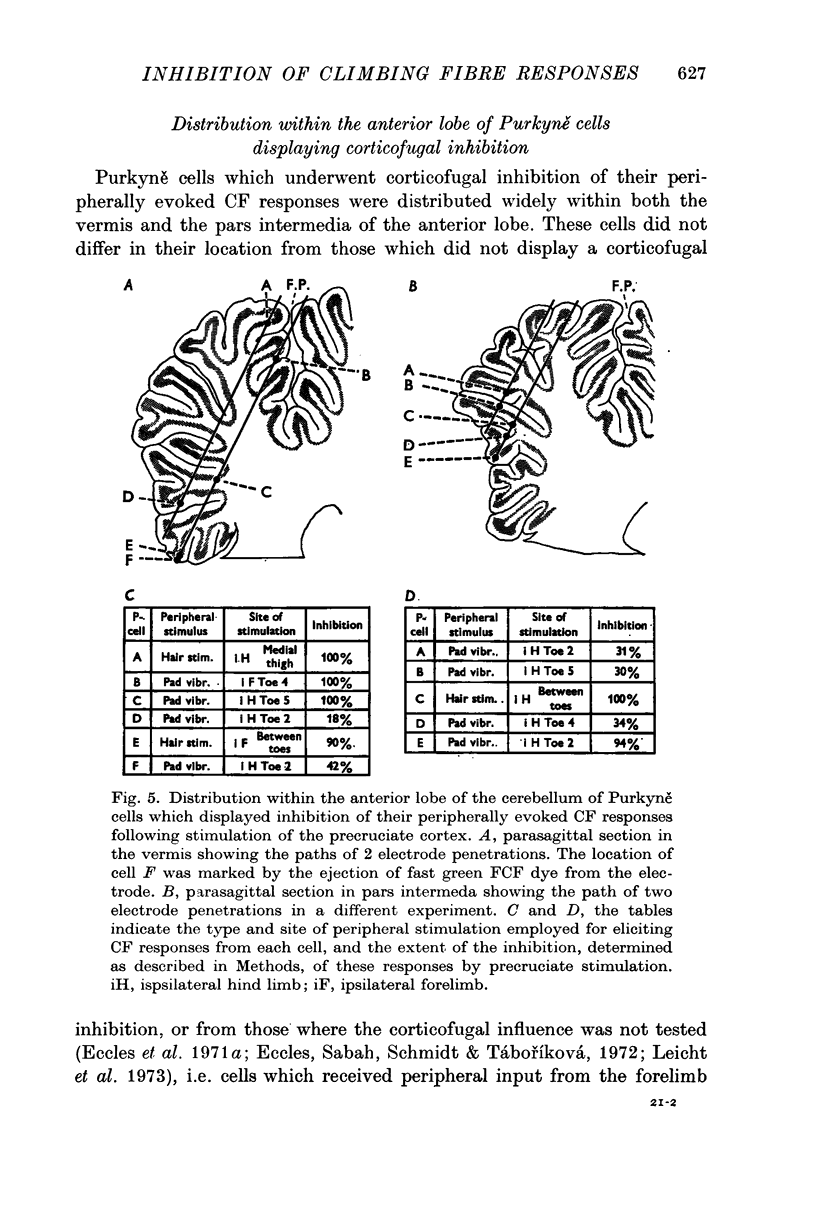
3. In most cases the corticofugal inhibition of cutaneously evoked CF responses was mediated by inhibitory mechanisms outside the cerebellar cortex, probably at relays within the spino-olivocerebellar pathways. Purkyně cells undergoing corticofugal inhibition were distributed widely within both the vermis and the pars intermedia of the anterior lobe.
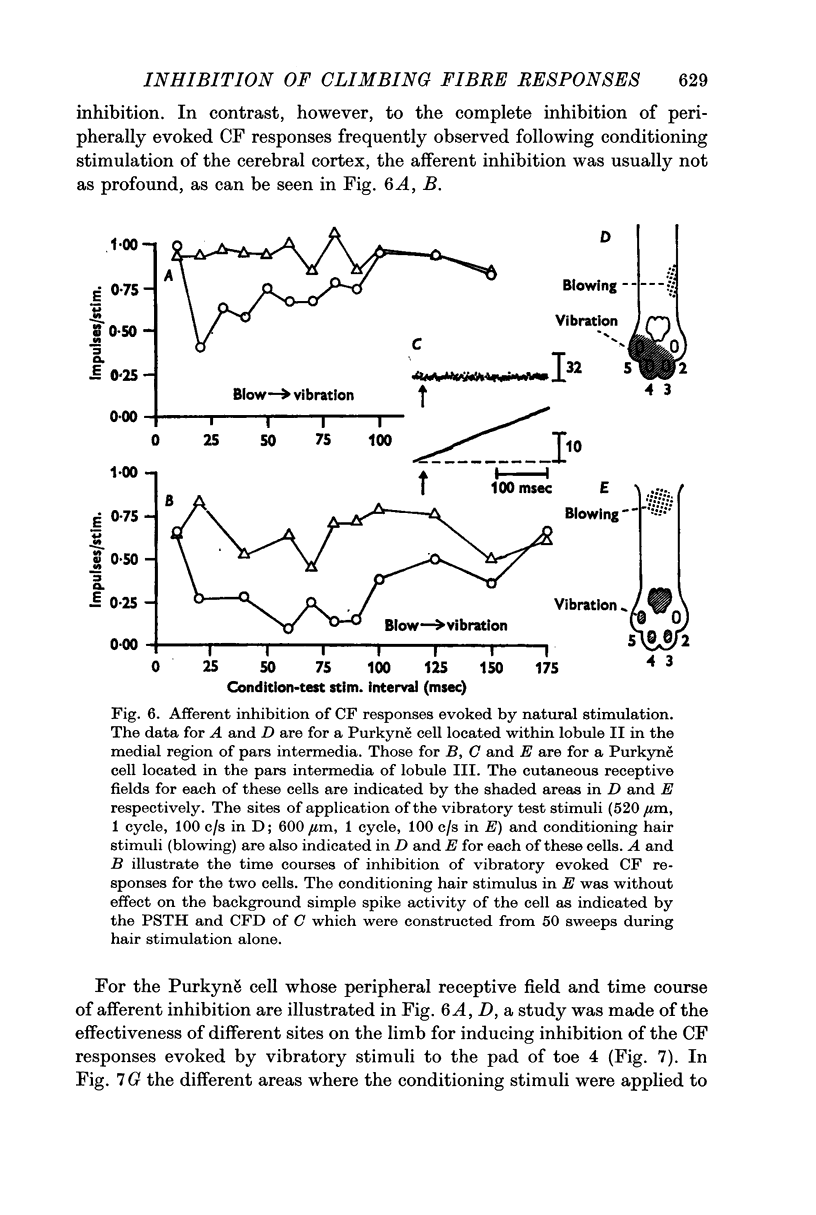
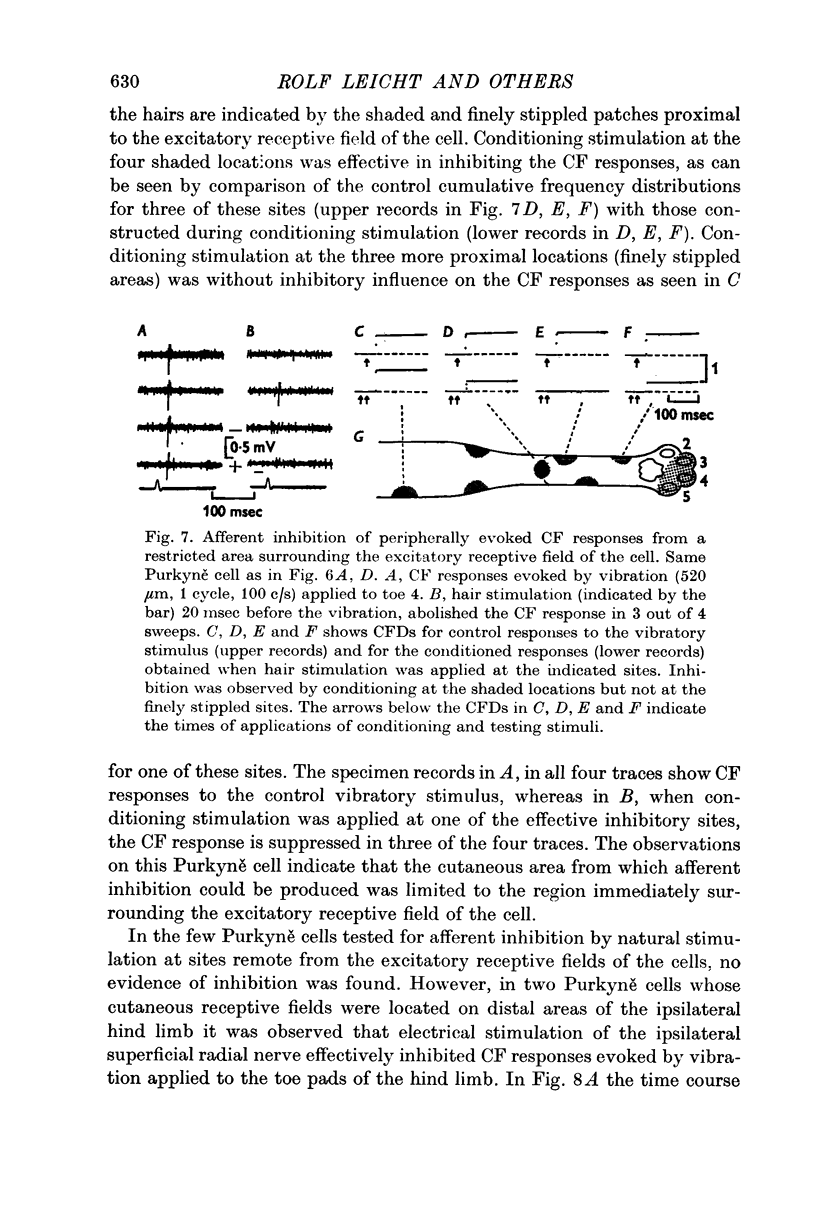
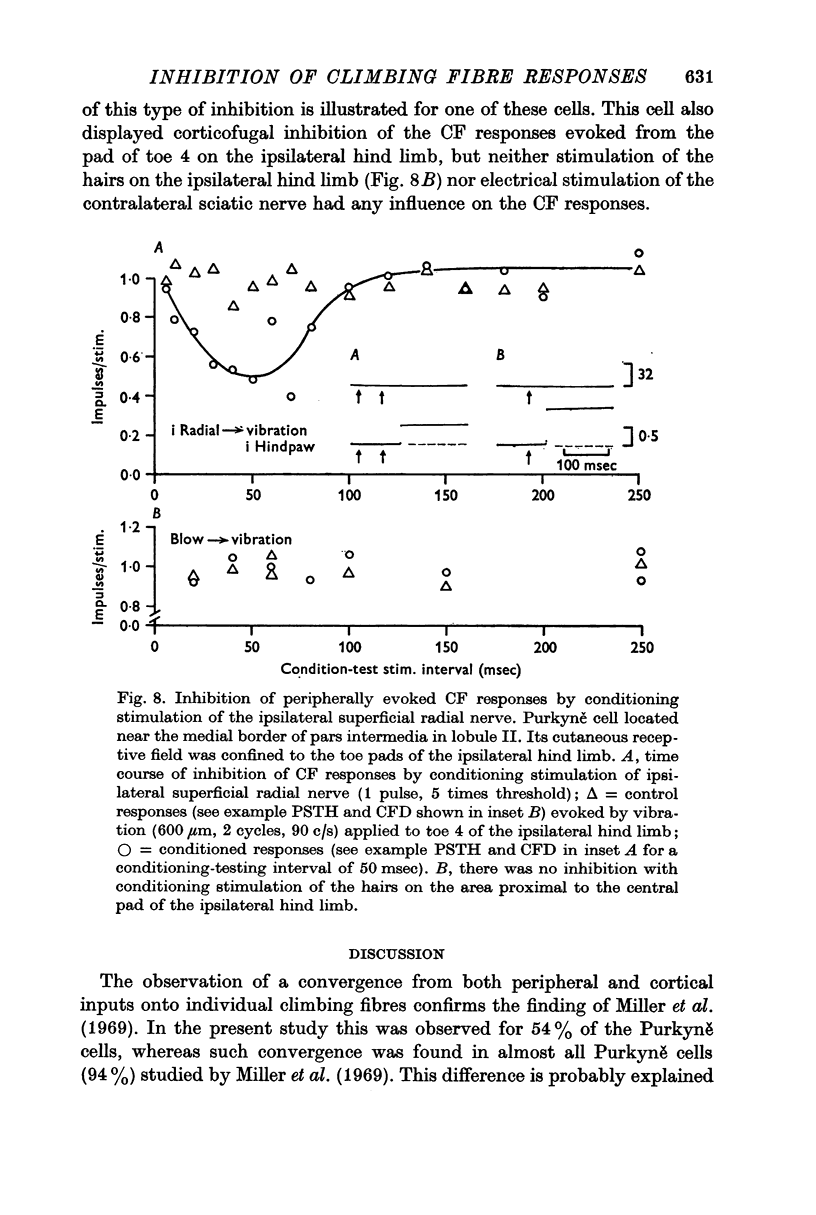
4. In 40% of all Purkyně cells tested, there was evidence for afferent inhibition of their peripherally evoked CF responses as revealed by conditioning stimuli applied to the skin outside the receptive field. Again it was found that the inhibition was exerted at levels prior to the cerebellum.
5. It is concluded that the afferent input transmitted to Purkyně cells via climbing fibres can be modified by corticofugal and peripheral influences exerted on the relays of the CF pathways outside the cerebellar cortex.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B. D., Harvey R. J. Responses in the inferior olive to stimulation of the cerebellar and cerebral cortices in the cat. J Physiol. 1966 Dec;187(3):553–574. doi: 10.1113/jphysiol.1966.sp008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill W. E., Kennedy T. T. Inferior olive of the cat: intracellular recording. Science. 1967 Aug 11;157(3789):716–718. doi: 10.1126/science.157.3789.716. [DOI] [PubMed] [Google Scholar]
- Crill W. E. Unitary multiple-spiked responses in cat inferior olive nucleus. J Neurophysiol. 1970 Mar;33(2):199–209. doi: 10.1152/jn.1970.33.2.199. [DOI] [PubMed] [Google Scholar]
- Ebbesson S. O. A connection between the dorsal column nuclei and the dorsal accessory alive. Brain Res. 1968 May;8(2):393–397. doi: 10.1016/0006-8993(68)90062-0. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Afferent volleys in limb nerves influencing impulse discharges in cerebellar cortex. II. In Purkyne cells. Exp Brain Res. 1971 Jul 26;13(1):36–53. [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Murphy J. T., Sabah N. H., Táboríková H. Investigations on integration of mossy fiber inputs to Purkynè cells in the anterior lobe. Exp Brain Res. 1971 Jul 26;13(1):54–77. [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K., Voorhoeve P. E. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. J Physiol. 1966 Jan;182(2):297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Analysis of electrical potentials evoked in the cerebellar anterior lobe by stimulation of hindlimb and forelimb nerves. Exp Brain Res. 1968;6(3):171–194. doi: 10.1007/BF00235123. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Integration by Purkyne cells of mossy and climbing fiber inputs from cutaneous mechanoreceptors. Exp Brain Res. 1972 Oct 29;15(5):498–520. doi: 10.1007/BF00236405. [DOI] [PubMed] [Google Scholar]
- Freeman J. A. Responses of cat cerebellar Purkinje cells to convergent inputs from cerebral cortex and peripheral sensory systems. J Neurophysiol. 1970 Nov;33(6):697–712. doi: 10.1152/jn.1970.33.6.697. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. Correlation of different excitatory and inhibitory influences on cells in the nucleus gracilis of the cat. Nature. 1962 Dec 22;196:1183–1185. doi: 10.1038/1961183a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Kawaguchi S., Rowe M. J. Actions of afferent impulses from muscle receptors on cerebellar Purkyne cells. I. Responses to muscle vibration. Exp Brain Res. 1972;15(2):177–193. doi: 10.1007/BF00235581. [DOI] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. J Physiol. 1969 Aug;203(3):611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham A., Paul D. H. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971 Feb;213(1):135–156. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht R., Roowe M. J., Schmidt R. F. Inhibition of cerebellar climbing fibre activity by stimulation of precruciate cortex. Brain Res. 1972 Aug 25;43(2):640–644. doi: 10.1016/0006-8993(72)90421-0. [DOI] [PubMed] [Google Scholar]
- Miller S., Nezlina N., Oscarsson O. Projection and convergence patterns in climbing fibre paths to cerebellar anterior lobe activated from cerebral cortex and spinal cord. Brain Res. 1969 Jun;14(1):230–233. doi: 10.1016/0006-8993(69)90045-6. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969 Jan;200(1):129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the ventral spino-olivocerebellar path. J Physiol. 1968 May;196(2):453–478. doi: 10.1113/jphysiol.1968.sp008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provini L., Redman S., Strata P. Mossy and climbing fibre organization on the anterior lobe of the cerebellum activated by forelimb and hindlimb areas of the sensorimotor cortex. Exp Brain Res. 1968;6(3):216–233. doi: 10.1007/BF00235125. [DOI] [PubMed] [Google Scholar]


