Abstract
1. The membrane potential of the separated longitudinal muscle of the guinea-pig terminal ileum was recorded intracellularly with glass micro-electrodes.
2. In tissues kept at room temperature and then brought to 35° C for 15-30 min or about 1 hr, the fall in membrane potential upon changing to potassium-free solution was 21.4 ± 3.5 mV and 13.4 ± 1.8 mV respectively. Ouabain (1.7 × 10-6 M) produced a fall in membrane potential of 8.1 ± 1.1 mV. Returning potassium to potassium-free solution, or changing from ouabain-containing to ouabain-free solution, resulted in an increase in membrane potential which was greater than the initial fall.
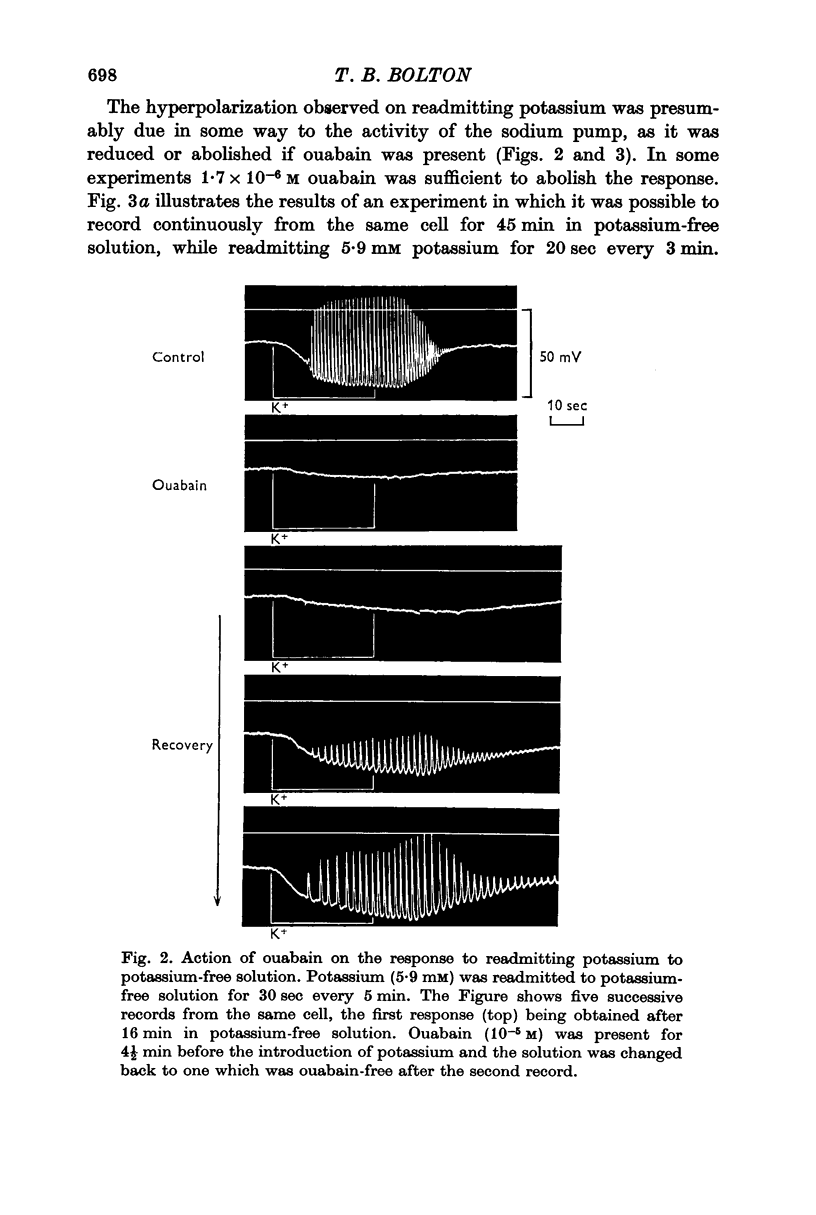
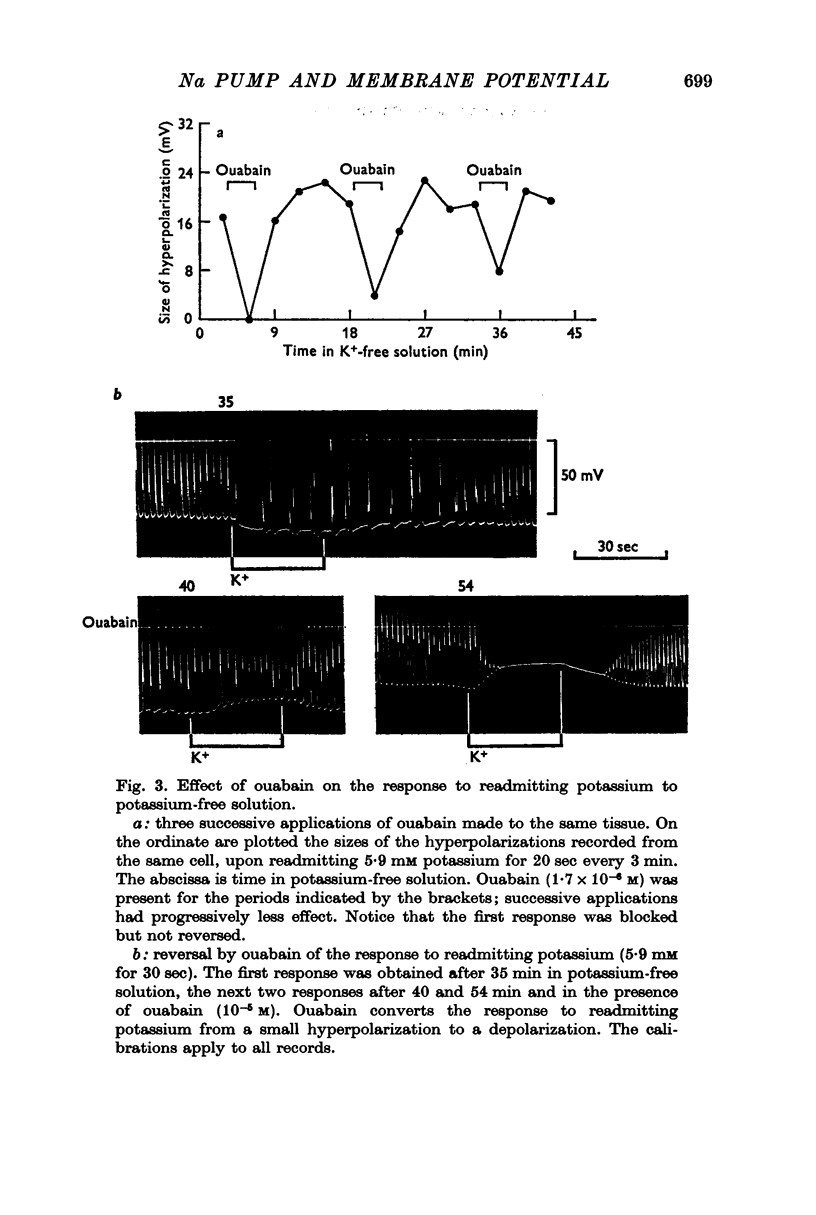
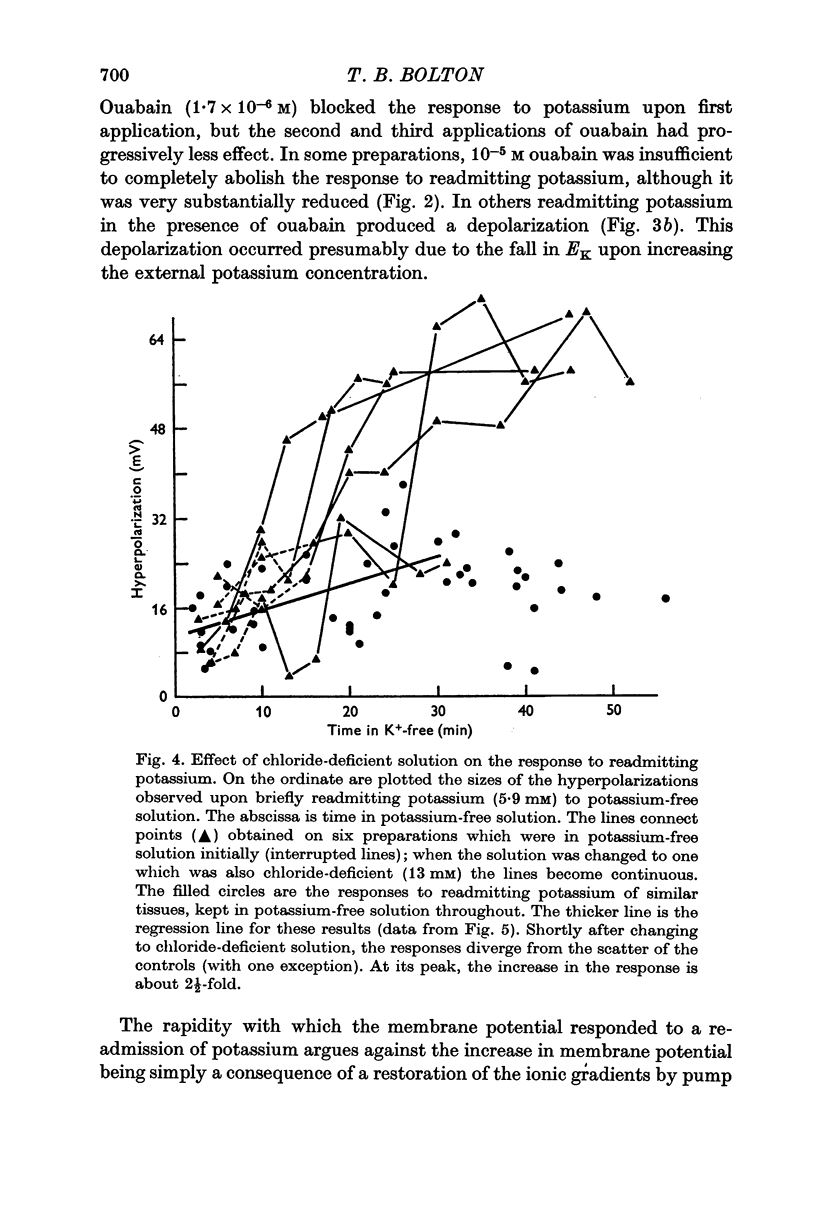
3. Readmitting potassium to potassium-free solution produced an increase in membrane potential which began within 10 sec and reached a maximum within 15-30 sec. This response was reduced, abolished, or converted to a depolarization by ouabain. In chloride-deficient (13 mM) solution in which membrane resistance was increased, the response to readmitting potassium was increased 2½-fold so that the membrane potential sometimes exceeded -100 mV, which was probably more negative than EK. On the basis of these results it was assumed that the response to readmitting potassium was due to the electrogenic activity of the sodium pump.
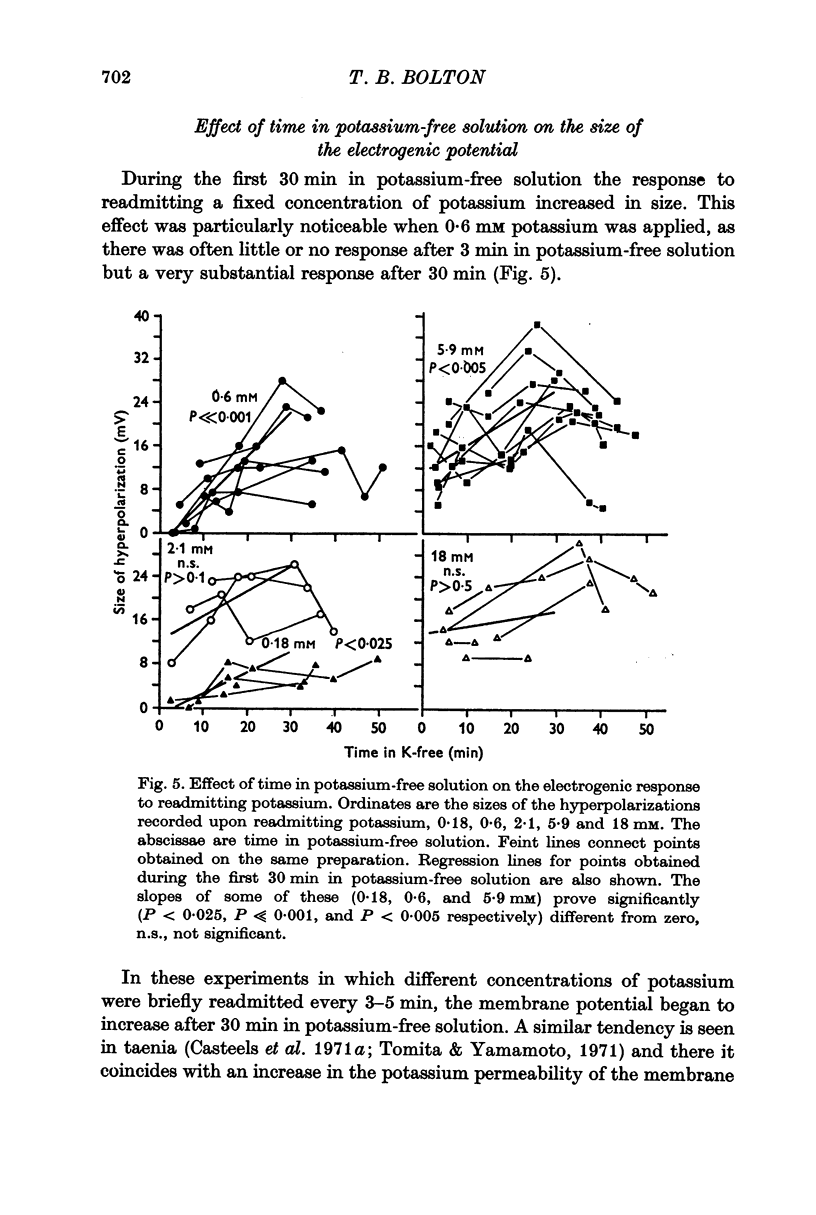
4. The response to briefly readmitting a fixed concentration of potassium increased during the first 30 min in potassium-free solution. This increase was not due to an increase in membrane resistance as this fell with time in potassium-free solution. It was suggested that the increase in the response resulted from the progressive rise in internal sodium concentration which is known to occur in smooth muscle in potassium-free solution.
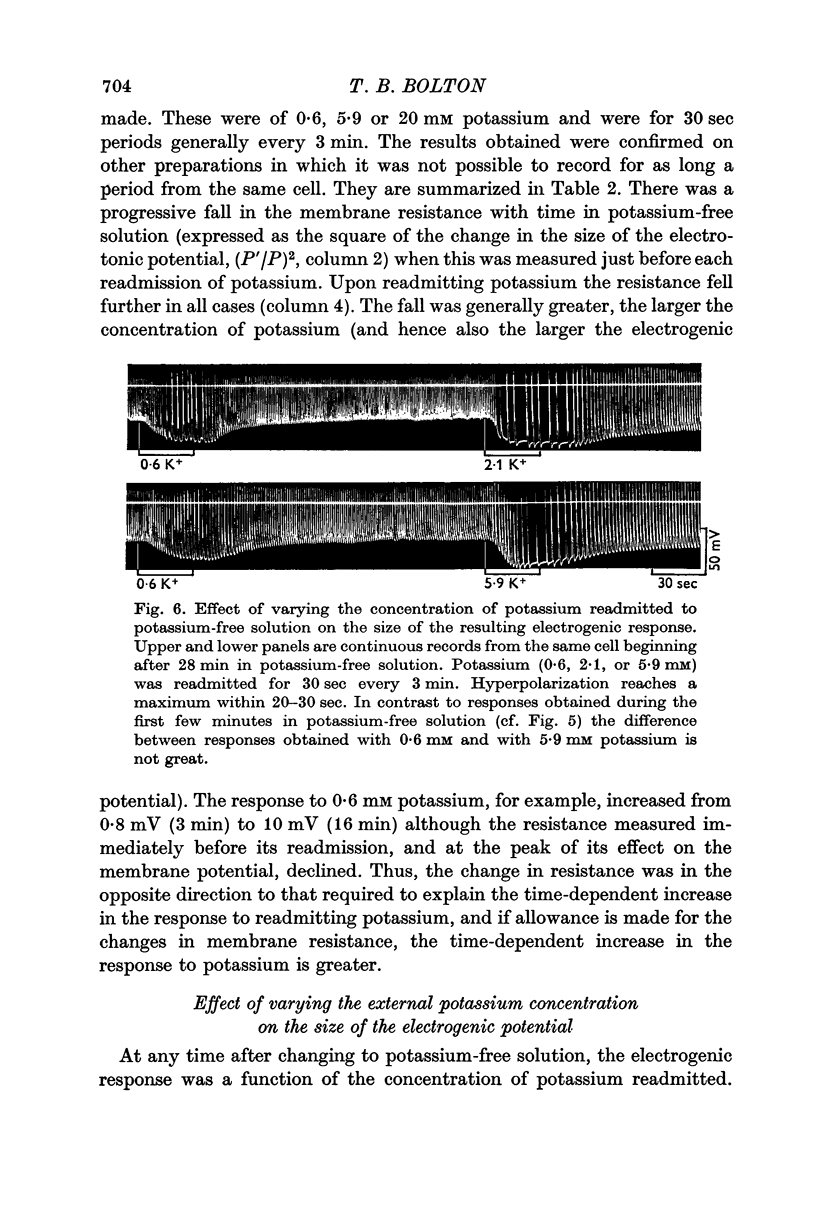
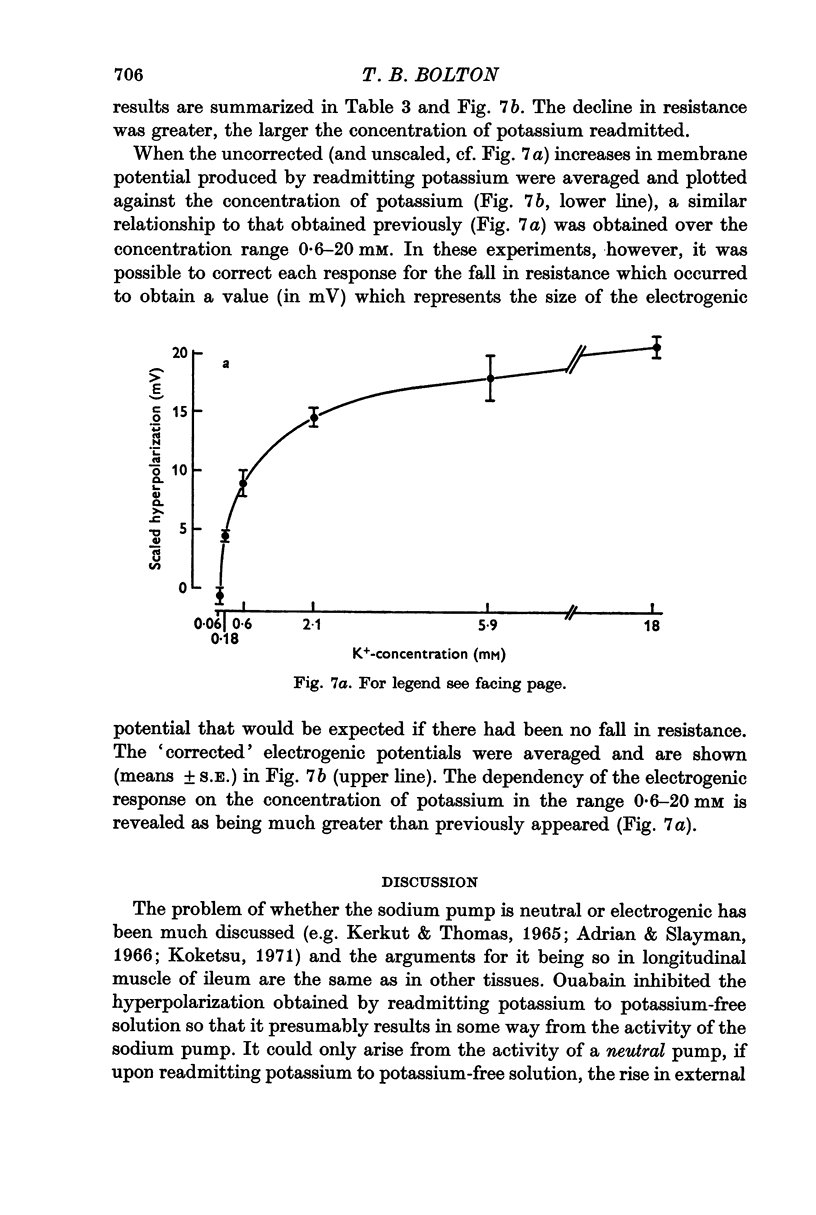
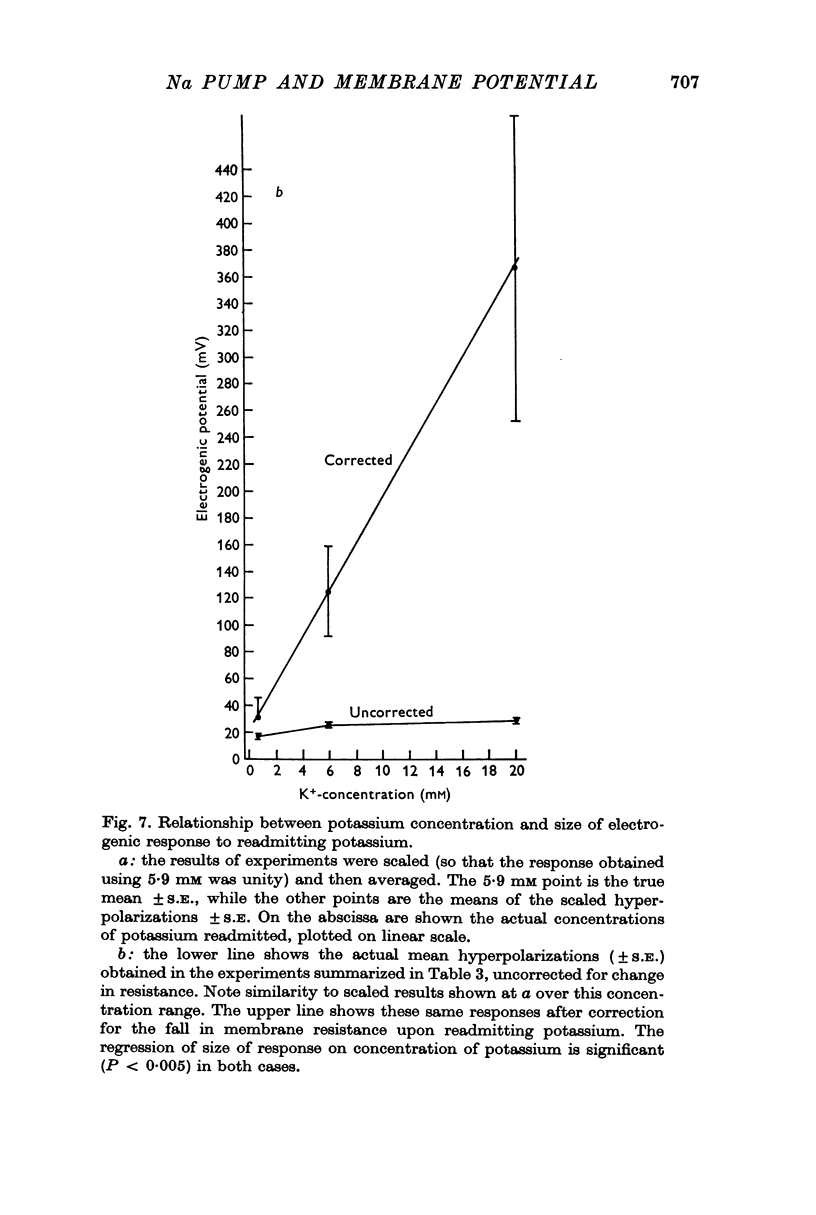
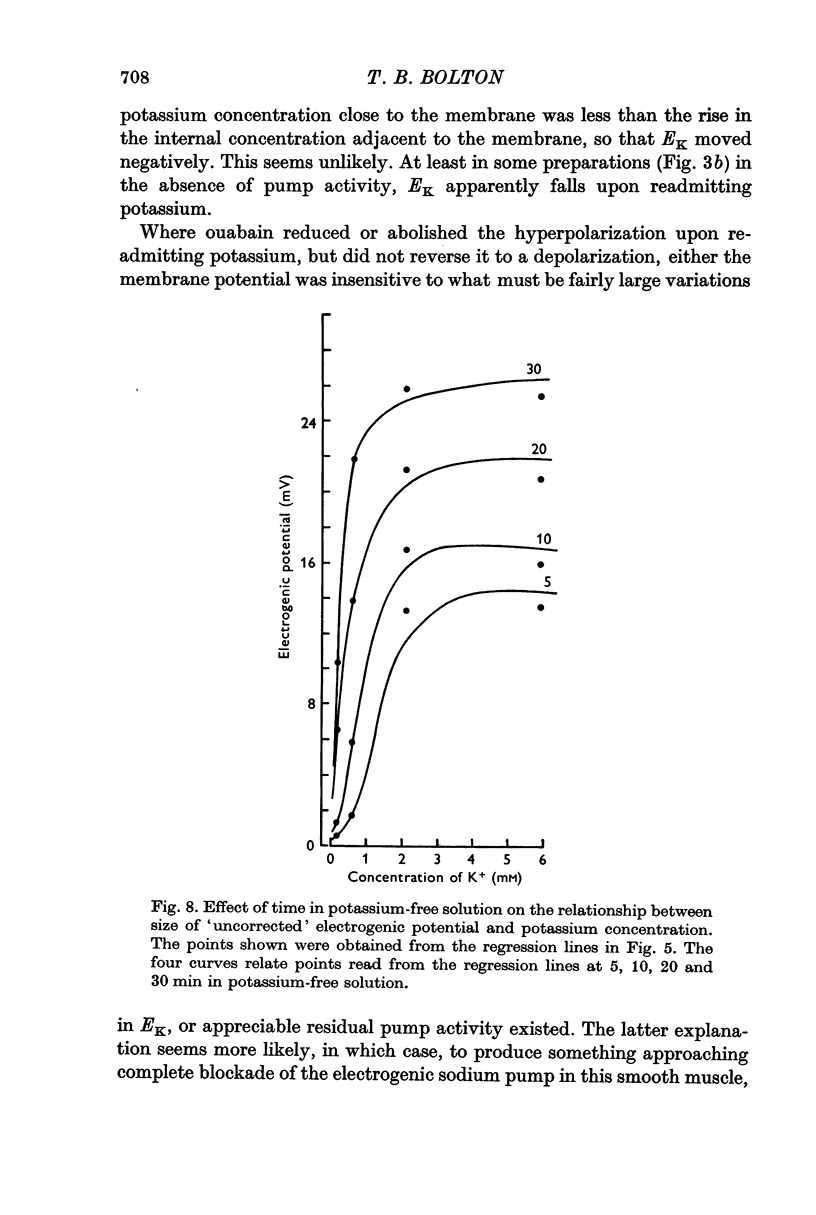
5. Increasing the concentration of potassium over the range ∼ 0.1-20 mM, increased the size of the electrogenic potential observed upon readmitting potassium to potassium-free solution. There was a fall in membrane resistance upon readmitting potassium (0.6, 5.9, or 20 mM) which was greater the larger the concentration of potassium. When allowance was made for the fall in membrane resistance, the dependency of the electrogenic response upon the concentration of potassium over the range 0.6-20 mM was much increased.
6. The results indicate that the rate of electrogenic sodium pumping in this tissue is increased by increasing the external potassium concentration, and probably by increasing the internal sodium concentration. It was suggested that a rise in the latter could sensitize the pump to an increase in the former.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J., Holmberg B. The effects of K plus -free solution on tension development in the smooth muscle taenia coli from the guinea pig. Acta Physiol Scand. 1971 Jul;82(3):322–332. doi: 10.1111/j.1748-1716.1971.tb04973.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Electrophysiological evidence of an electrogenic sodium pump in the longitudinal muscle of guinea-pig ileum and its involvement in the response to acetylcholine. J Physiol. 1971 Oct;218 (Suppl):58P–59P. [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Casteels R., Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. Br J Pharmacol. 1968 Oct;34(2):388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Electrogenic sodium pump in smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Sep;217(2):297–313. doi: 10.1113/jphysiol.1971.sp009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Hendrickx H. Pompe à sodium électrogène dans les fibres lisses du taenia coli de cobaye. J Physiol (Paris) 1969;61 (Suppl 2):240–240. [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The action of ouabain on the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1966 May;184(1):131–142. doi: 10.1113/jphysiol.1966.sp007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., MAIZELS M. The permeability of human erythrocytes to sodium. J Physiol. 1951 May;113(4):506–524. doi: 10.1113/jphysiol.1951.sp004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Taylor J. W., Waggoner D. M. Fractionation of sodium effux in frog sartorius muscles by strophanthidin and removal of external sodium. J Gen Physiol. 1970 Mar;55(3):401–425. doi: 10.1085/jgp.55.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K. The electrogenic sodium pump. Adv Biophys. 1971;2:77–112. [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi H. An estimate of the proportion of the resting membrane conductance of the smooth muscle of guinea-pog taenia coli attributable to chloride. J Physiol. 1970 Sep;210(2):405–419. doi: 10.1113/jphysiol.1970.sp009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Effect of rubidium and caesium on electrogenic sodium pumping in rat myometrium. Comp Biochem Physiol A Comp Physiol. 1971 Feb 1;38(2):251–264. doi: 10.1016/0300-9629(71)90052-1. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Evidence for an electrogenic sodium pump in smooth muscle. Life Sci. 1969 Jul 1;8(13):769–773. doi: 10.1016/0024-3205(69)90268-9. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog A., Ritchie J. M. A comparison of the effect of temperature, metabolic inhibitors and of ouabain on the electrogenic componen of the sodium pump in mammalian non-myelinated nerve fibres. J Physiol. 1969 Oct;204(3):523–538. doi: 10.1113/jphysiol.1969.sp008929. [DOI] [PMC free article] [PubMed] [Google Scholar]


