Abstract
1. Intracellular recording of membrane potential was made from the separated longitudinal muscle of the guinea-pig terminal ileum in physiological salt solution.
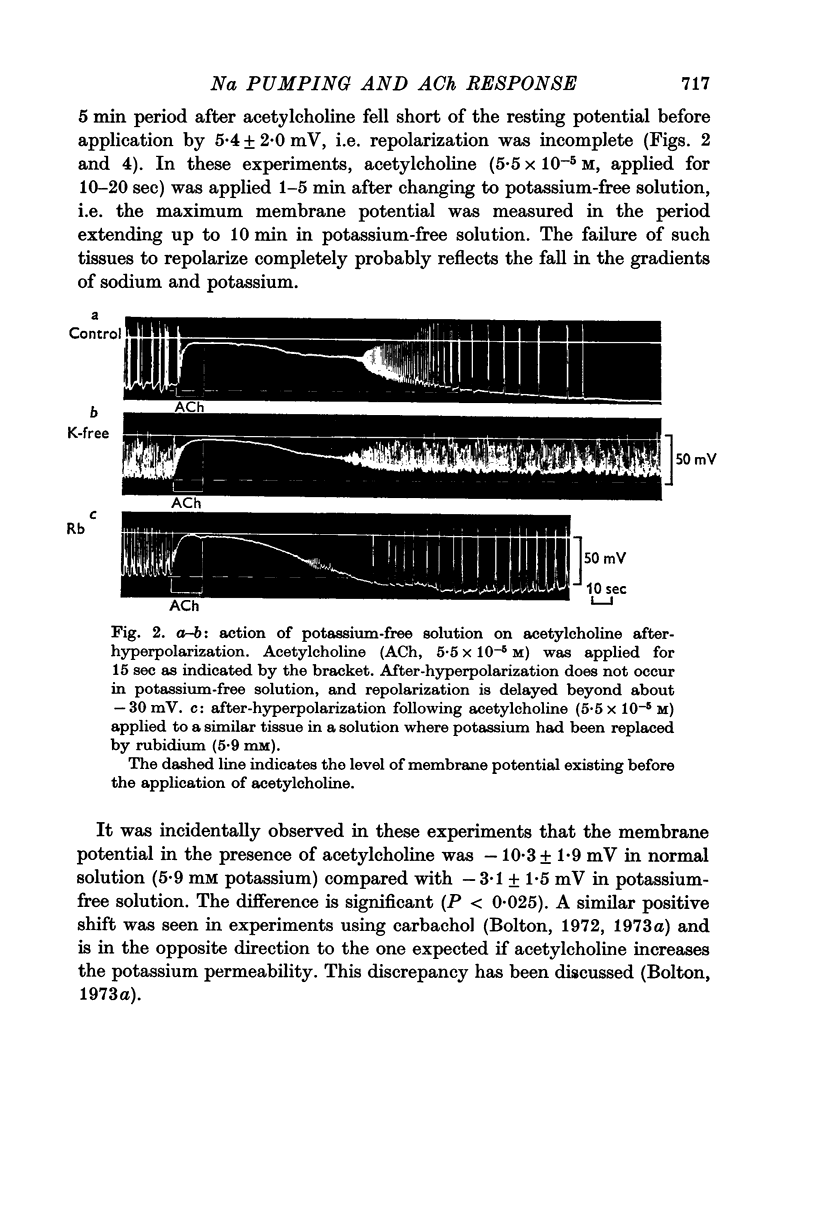
2. When acetylcholine was washed from the tissue following a brief application the membrane repolarized and then hyperpolarized (`after-hyperpolarization') beyond the level existing before the application of acetylcholine.
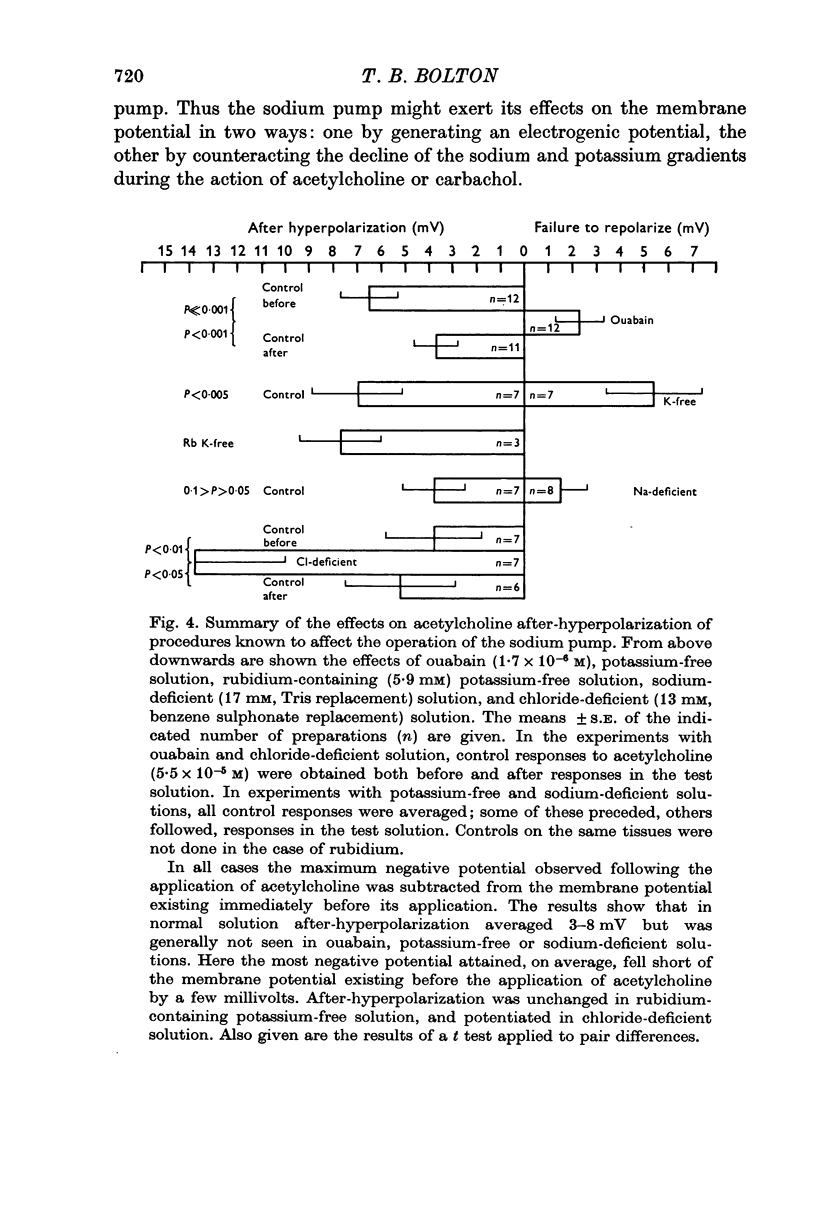
3. No after-hyperpolarization was observed following acetylcholine in potassium-free solution, in sodium-deficient (17 mM) solution, or in the presence of ouabain (1.7 × 10-6 M). Repolarization under these conditions was delayed, especially after the membrane potential reached -20 to -30 mV, and was generally incomplete.
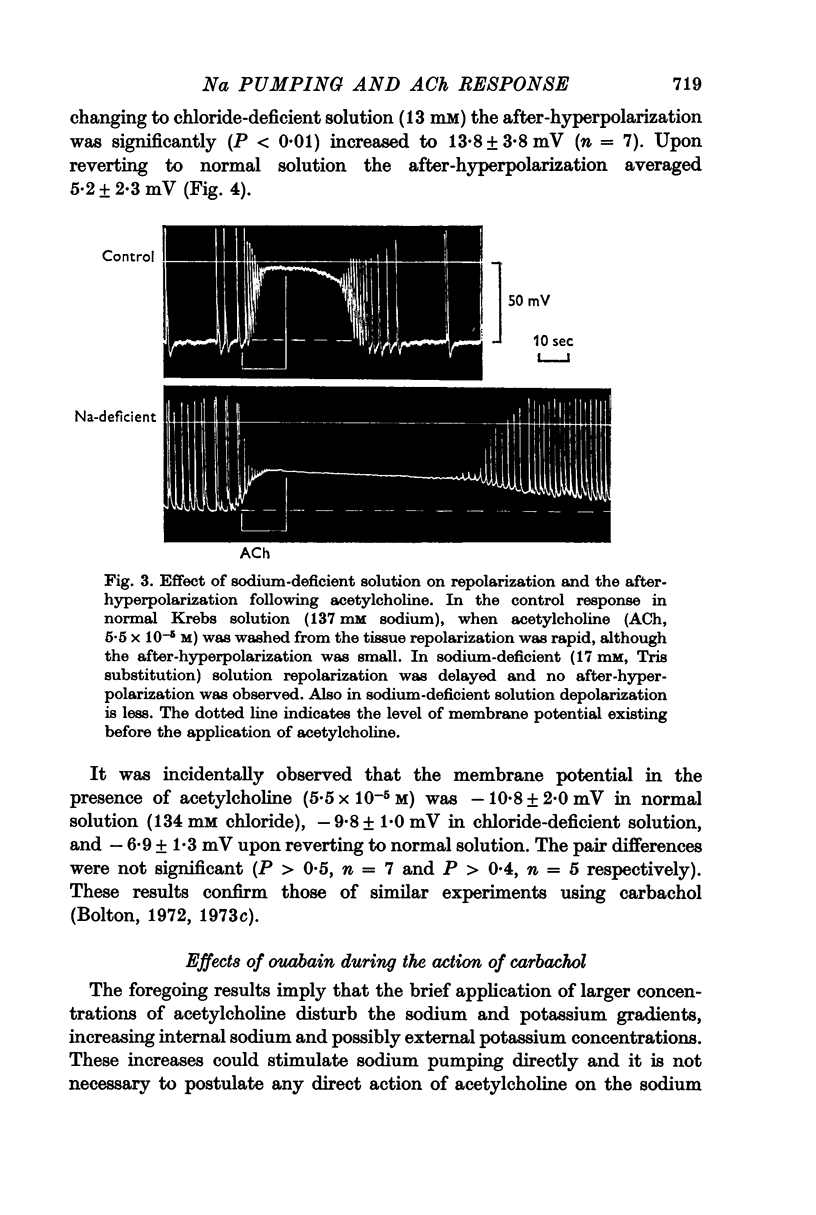
4. The after-hyperpolarization was significantly (P < 0.01) greater when acetylcholine was applied in chloride-deficient (13 mM) solution.
5. It was incidentally observed that the membrane potential in the presence of acetylcholine was more positive in potassium-free solution (significance P < 0.025), unchanged in chloride-deficient solution (P > 0.4), and much more negative in sodium-deficient (17 mM) solution (P ≪ 0.001), confirming previous results using carbachol.
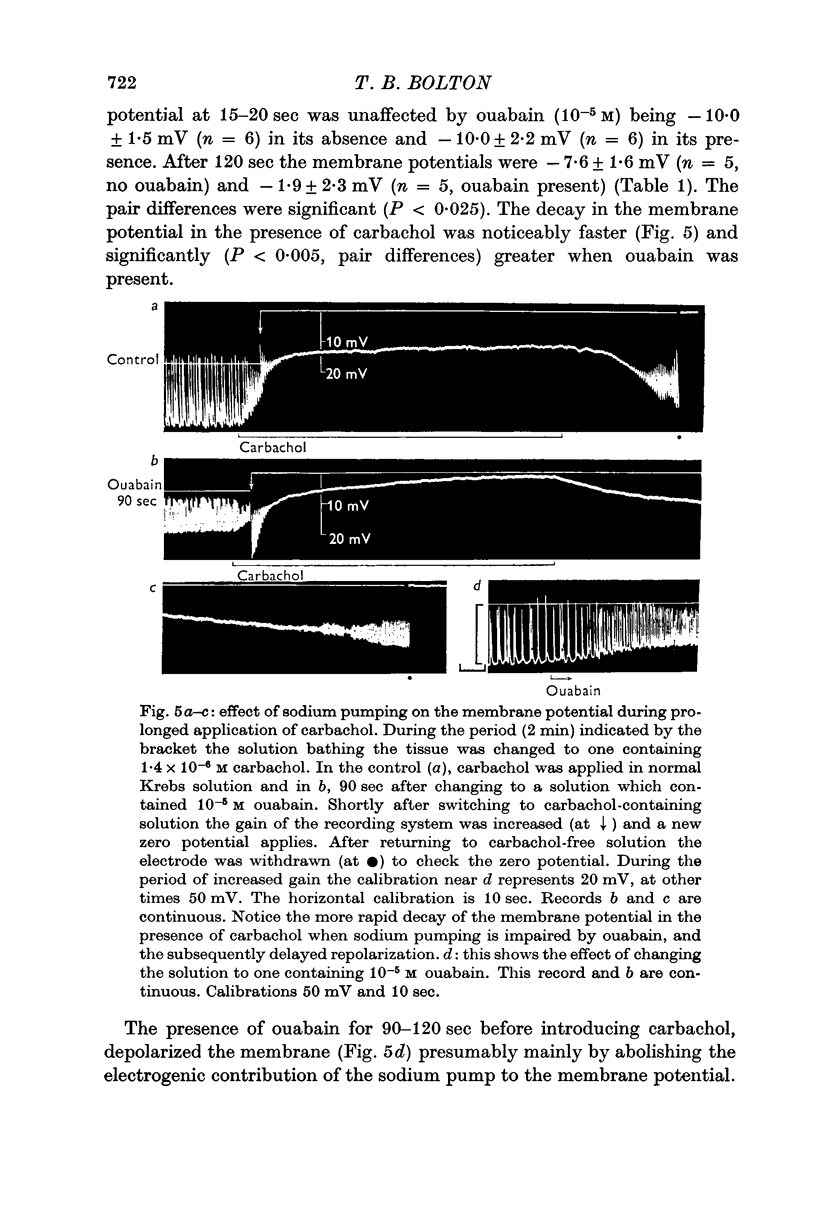
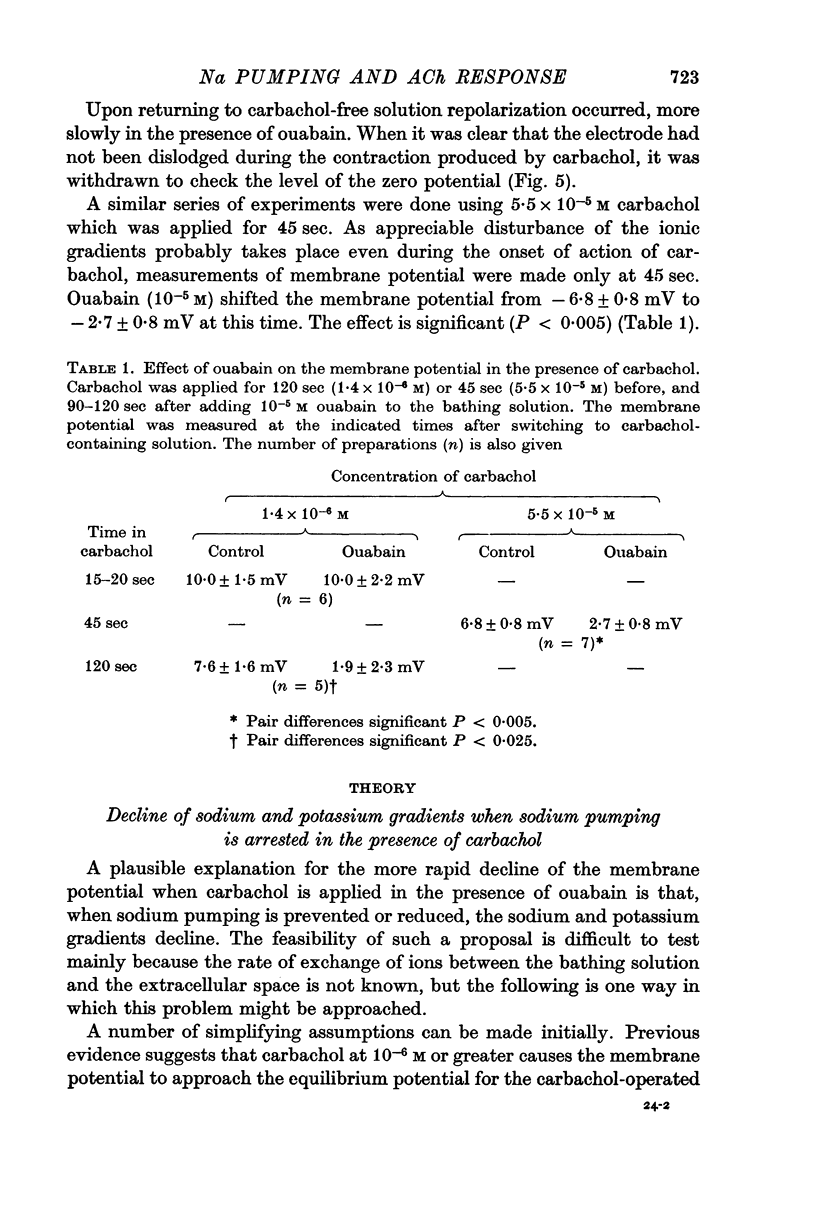
6. When a 2 min application of 1.4 × 10-6 M carbachol was made, the membrane potential 15-20 sec after beginning its application was not affected by ouabain (10-5 M), but showed a significantly (P < 0.005) greater positive shift subsequently, so that the potential after 120 sec in carbachol was significantly (P < 0.025) more positive in the presence of ouabain. After 45 sec in 5.5 × 10-5 M carbachol the membrane potential was also significantly (P < 0.005) more positive in the presence of ouabain (10-5 M).
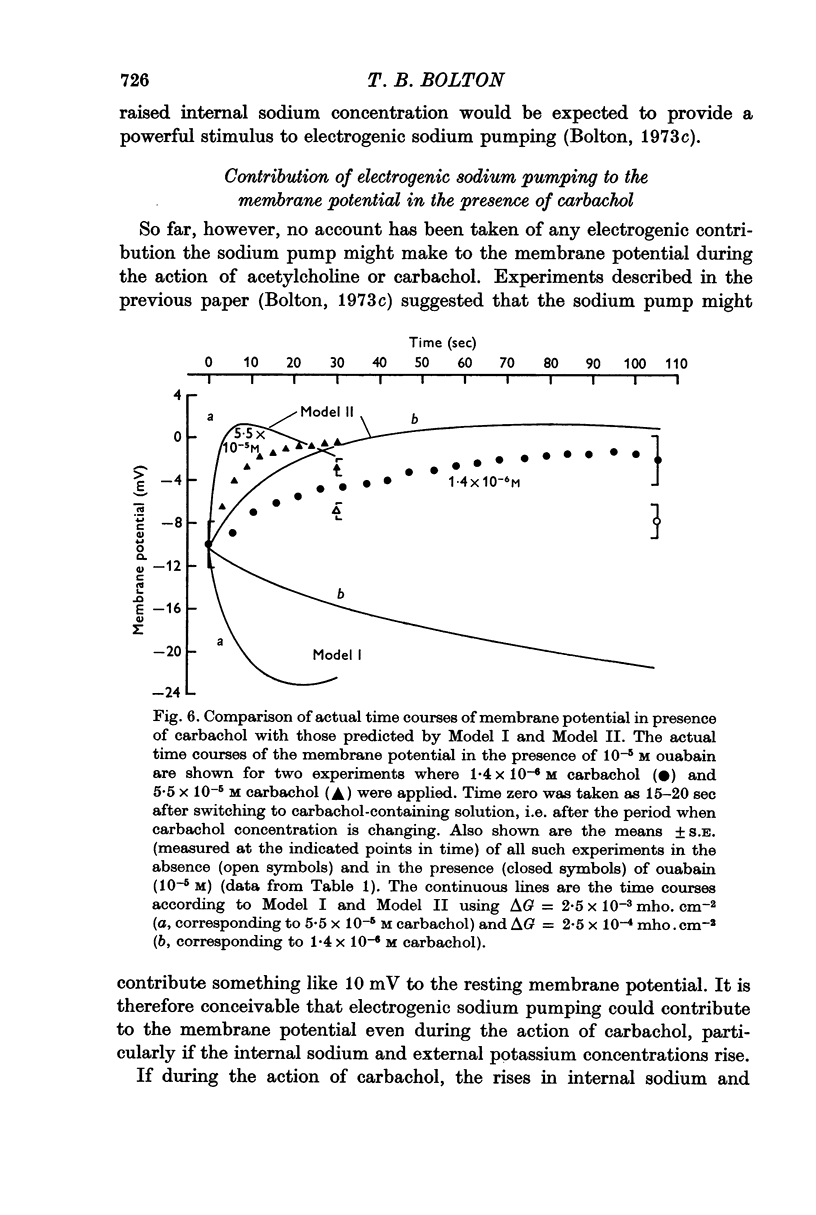
7. Calculations based on hypotheses concerning the movements of sodium and potassium showed that the positive shift of the membrane potential in the presence of carbachol when sodium pumping was arrested, could be quantitatively explained by a decline in the sodium and potassium gradients across the membrane. It appeared that the electrogenic fraction of the sodium pumped was small in the presence of carbachol.
8. It was concluded that the application of acetylcholine or carbachol (> 10-6 M) to this smooth muscle disturbs the sodium and potassium gradients across the membrane. These disturbances are in a direction which stimulates electrogenic sodium pumping. Some limitation of depolarization results, and the increased electrogenic extrusion of sodium is responsible for the after-hyperpolarization which follows the application of acetylcholine.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson J., Holmberg B. The effects of K plus -free solution on tension development in the smooth muscle taenia coli from the guinea pig. Acta Physiol Scand. 1971 Jul;82(3):322–332. doi: 10.1111/j.1748-1716.1971.tb04973.x. [DOI] [PubMed] [Google Scholar]
- BORN G. V., BULBRING E. The movement of potassium between smooth muscle and the surrounding fluid. J Physiol. 1956 Mar 28;131(3):690–703. doi: 10.1113/jphysiol.1956.sp005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in ionic environment on the action of acetylcholine and adrenaline on the smooth muscle cells of guinea-pig taenia coli. J Physiol. 1963 Apr;166:59–74. doi: 10.1113/jphysiol.1963.sp007090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The effects of acetylcholine on membrane potential, spike frequency, conduction velocity and excitability in the taenia coli of the guinea-pig. J Physiol. 1958 Aug 29;143(1):165–182. doi: 10.1113/jphysiol.1958.sp006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Effects of electrogenic sodium pumping on the membrane potential of longitudinal smooth muscle from terminal ileum of guinea-pig. J Physiol. 1973 Feb;228(3):693–712. doi: 10.1113/jphysiol.1973.sp010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Casteels R., Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. Br J Pharmacol. 1968 Oct;34(2):388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The action of ouabain on the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1966 May;184(1):131–142. doi: 10.1113/jphysiol.1966.sp007907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Kuriyama H. Responses of the smooth muscle membrane of guinea pig jejunum elicited by field stimulation. J Gen Physiol. 1969 Apr;53(4):471–486. doi: 10.1085/jgp.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMBECK F., STROBACH R. Kaliumabgabe aus glatter Muskulatur. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1956;228(1-2):130–131. [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. The binding of tritiated ouabain to mammalian non-myelinated nerve fibres. J Physiol. 1970 Apr;207(2):529–537. doi: 10.1113/jphysiol.1970.sp009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Sutter M. C. Ouabain-induced changes in the contractile and electrical activity, potassium content, and response to drugs, of smooth muscle cells. Can J Physiol Pharmacol. 1967 May;45(3):509–520. doi: 10.1139/y67-060. [DOI] [PubMed] [Google Scholar]
- PATON W. D., ROTHSCHILD A. M. THE CHANGES IN RESPONSE AND IN IONIC CONTENT OF SMOOTH MUSCLE PRODUCED BY ACETYLCHOLINE ACTON AND BY CALCIUM DEFICIENCY. Br J Pharmacol Chemother. 1965 Apr;24:437–448. doi: 10.1111/j.1476-5381.1965.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton D. M. Evidende for an effect of sodium pumping on spontaneous contractility of rabbit detrusor muscle. Comp Biochem Physiol A Comp Physiol. 1971 Nov 1;40(3):751–759. doi: 10.1016/0300-9629(71)90260-x. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Effect of rubidium and caesium on electrogenic sodium pumping in rat myometrium. Comp Biochem Physiol A Comp Physiol. 1971 Feb 1;38(2):251–264. doi: 10.1016/0300-9629(71)90052-1. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]


