Abstract
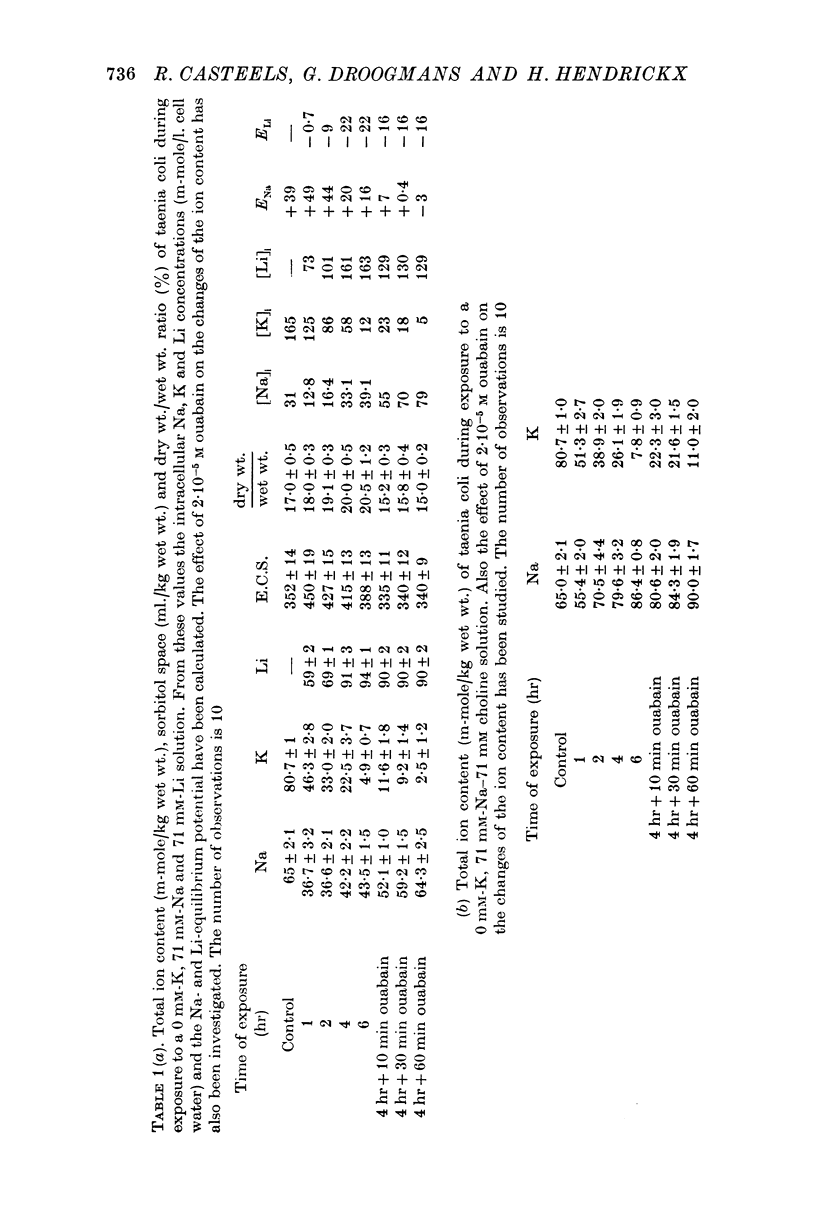
1. The changes of the ion content and of the membrane potential of taenia coli cells have been studied during prolonged exposure to Na-deficient solutions containing either Li or choline.
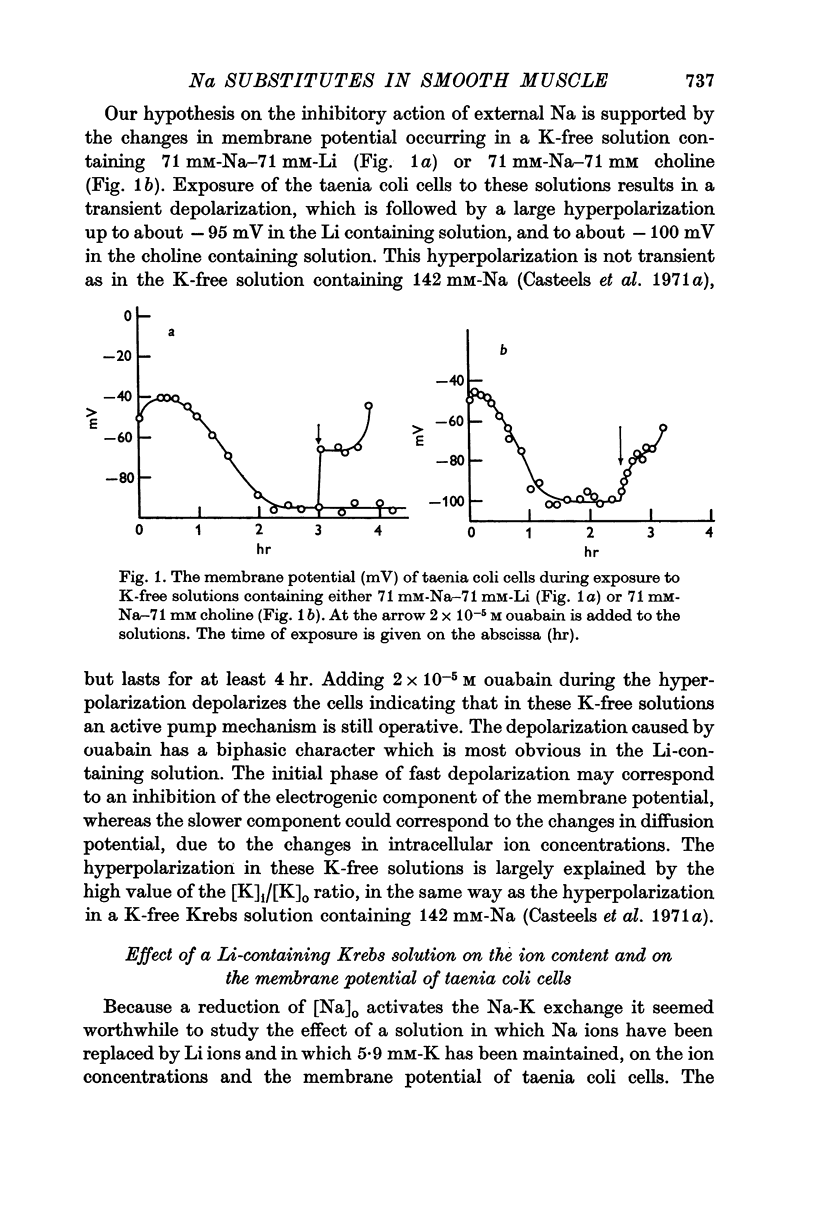
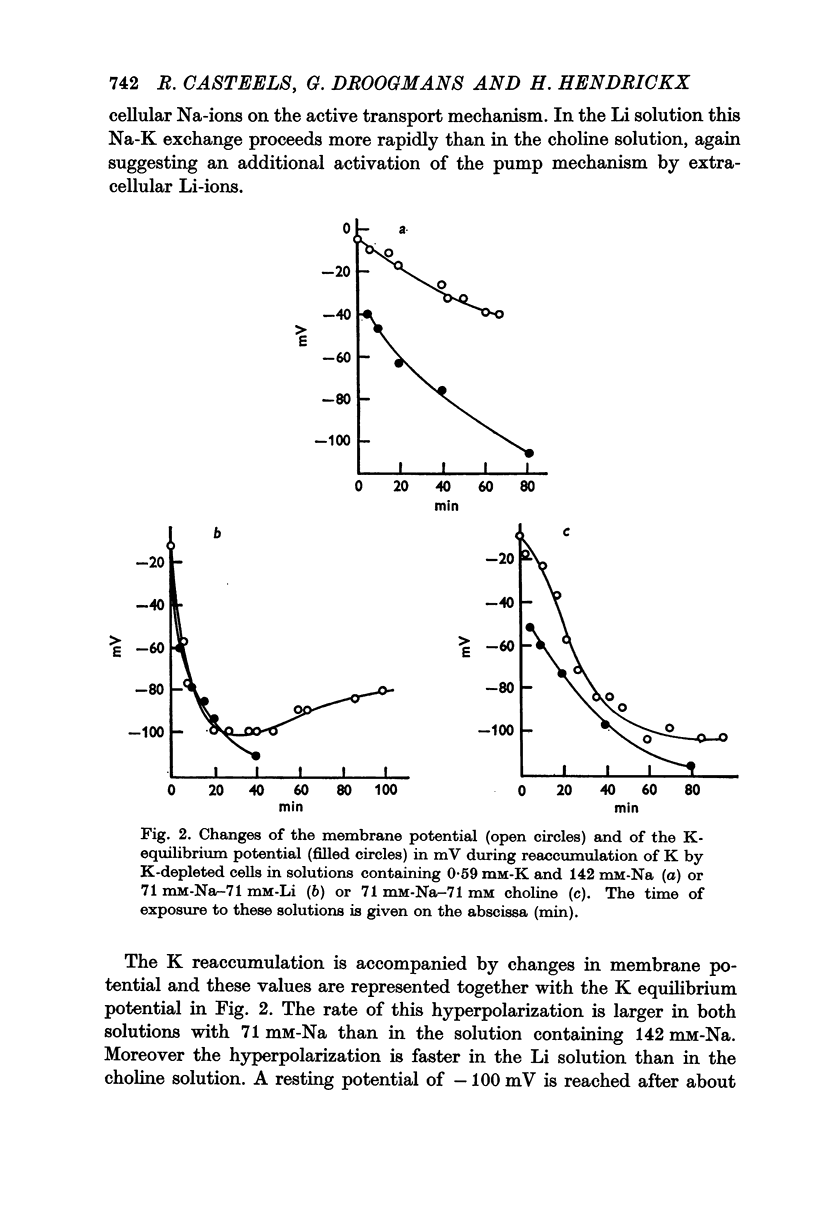
2. A K-free solution containing either 71 mM-Na-71 mM-Li or 71 mM-Na-71 mM choline causes a slower loss of cellular K than a 142 mM-Na solution. In both these Na-deficient solutions the membrane hyperpolarizes to about -100 mV for periods up to 6 hr. This hyperpolarization is partially abolished by 2 × 10-5 M ouabain.
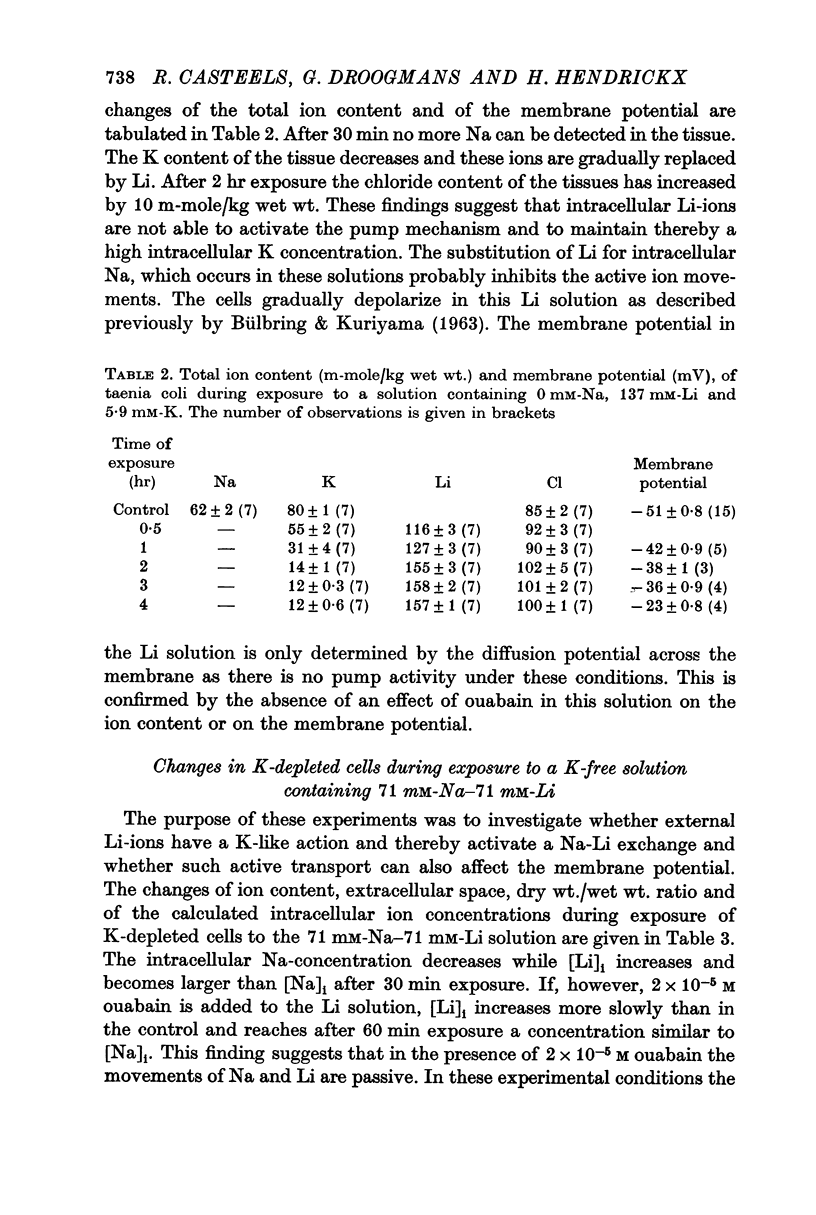
3. Replacing all extracellular Na by Li and maintaining 5.9 mM-K causes a fast loss of all Na and a progressive replacement of K by Li. These changes of the intracellular ion content are accompanied by a depolarization of the cells, suggesting that intracellular Li cannot substitute for Na in activating the ion pump.
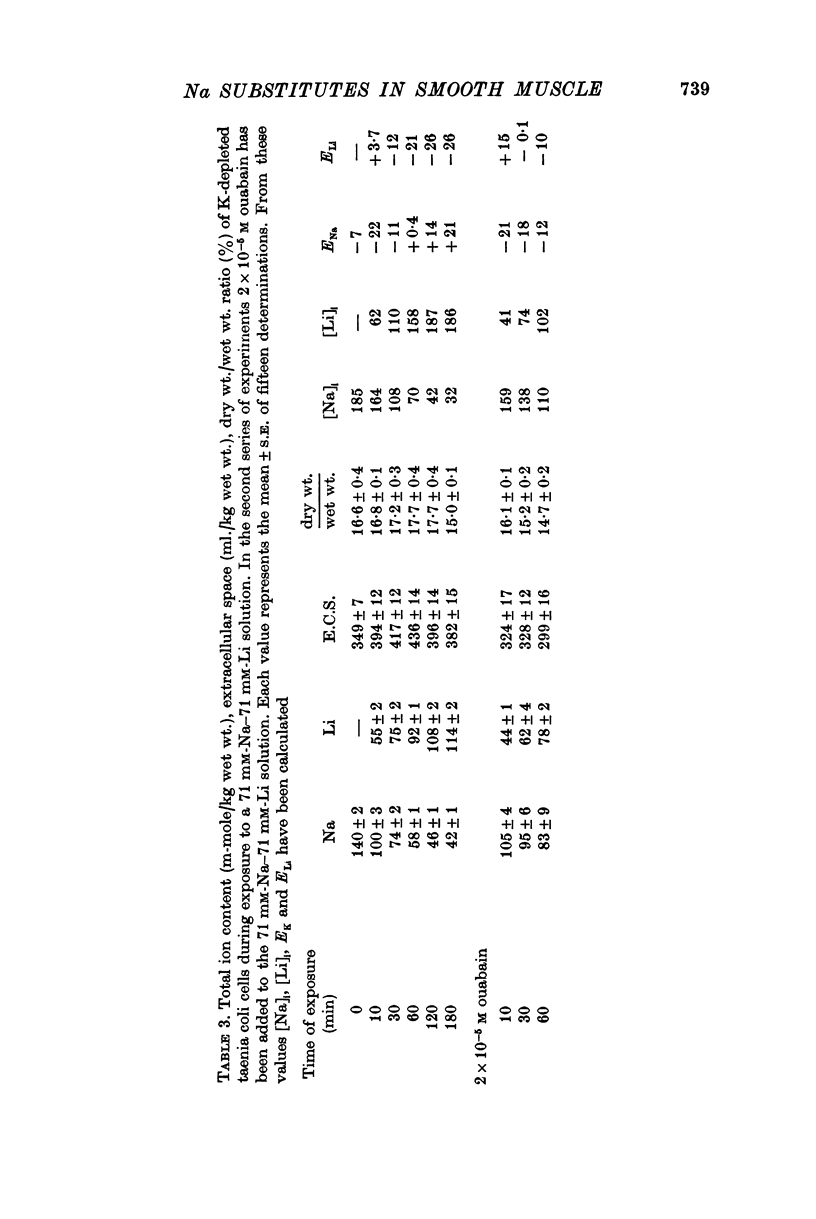
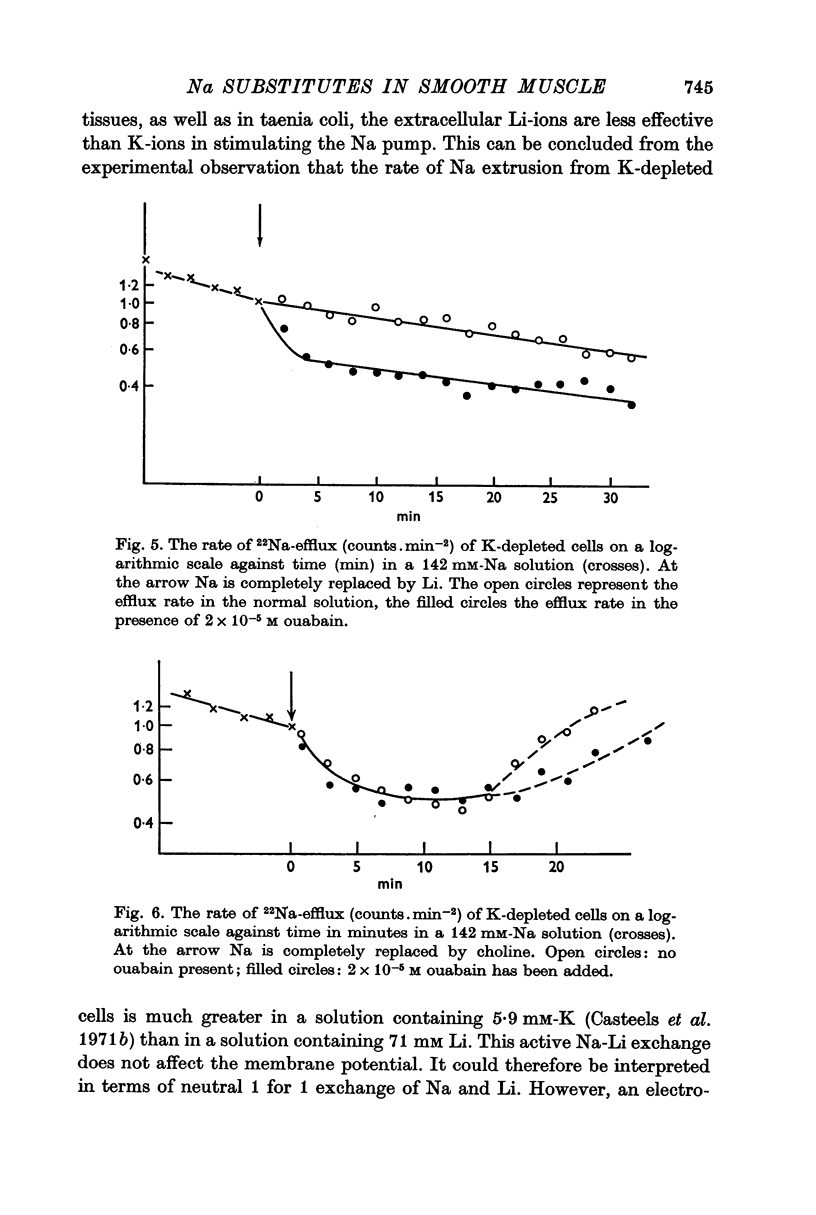
4. Exposing K-depleted cells to a K-free 71 mM-Na-71 mM-Li solution results in a ouabain sensitive transport of Na and Li against their electro-chemical gradient.
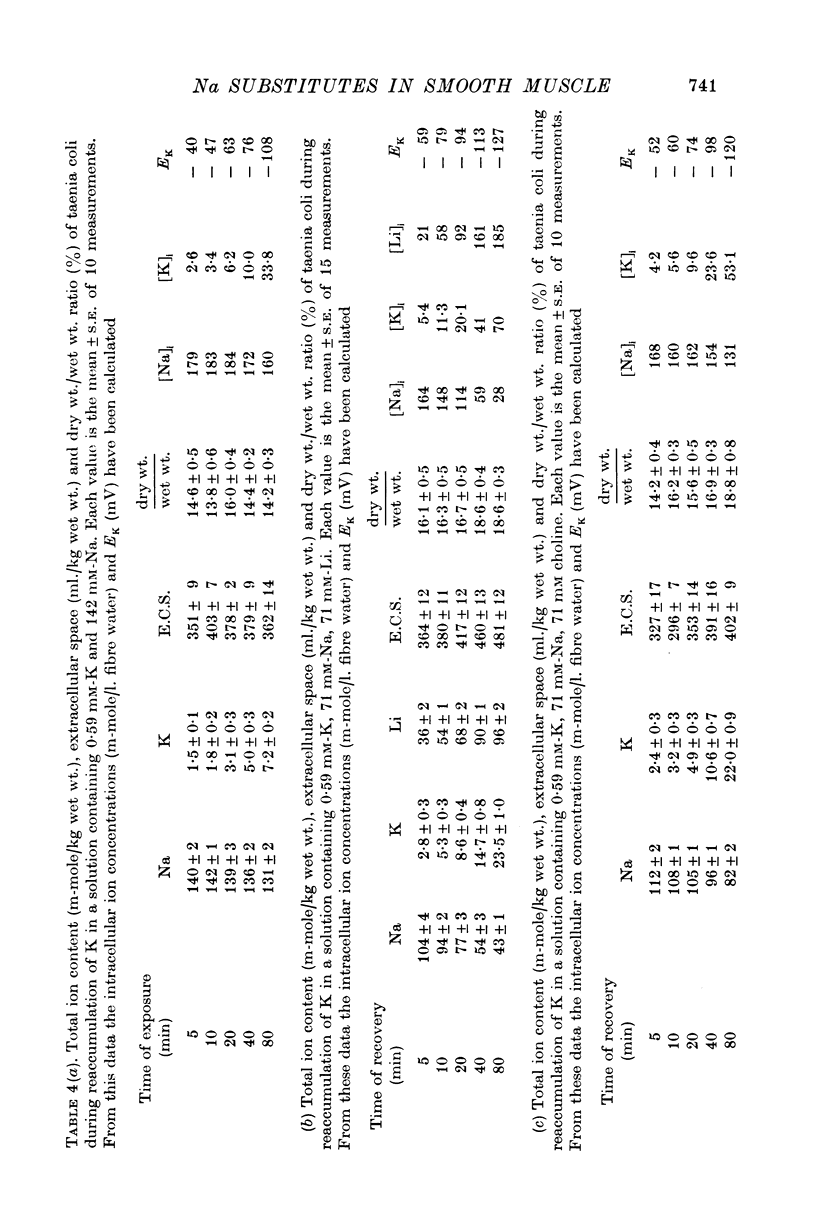
5. The K-uptake by K-depleted cells from a solution containing 0.59 mM-K is increased by reducing [Na]o to half of its normal value. This finding indicates that external Na inhibits the active Na-K exchange.
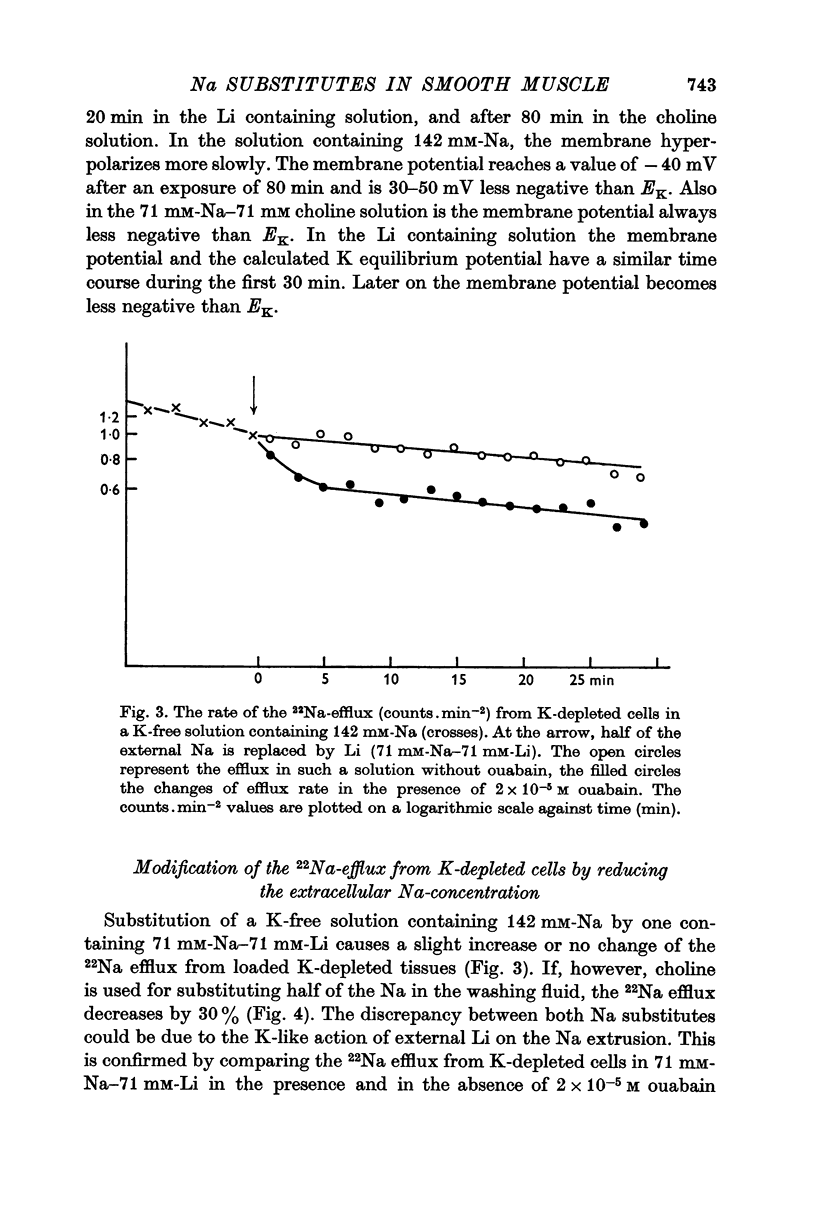
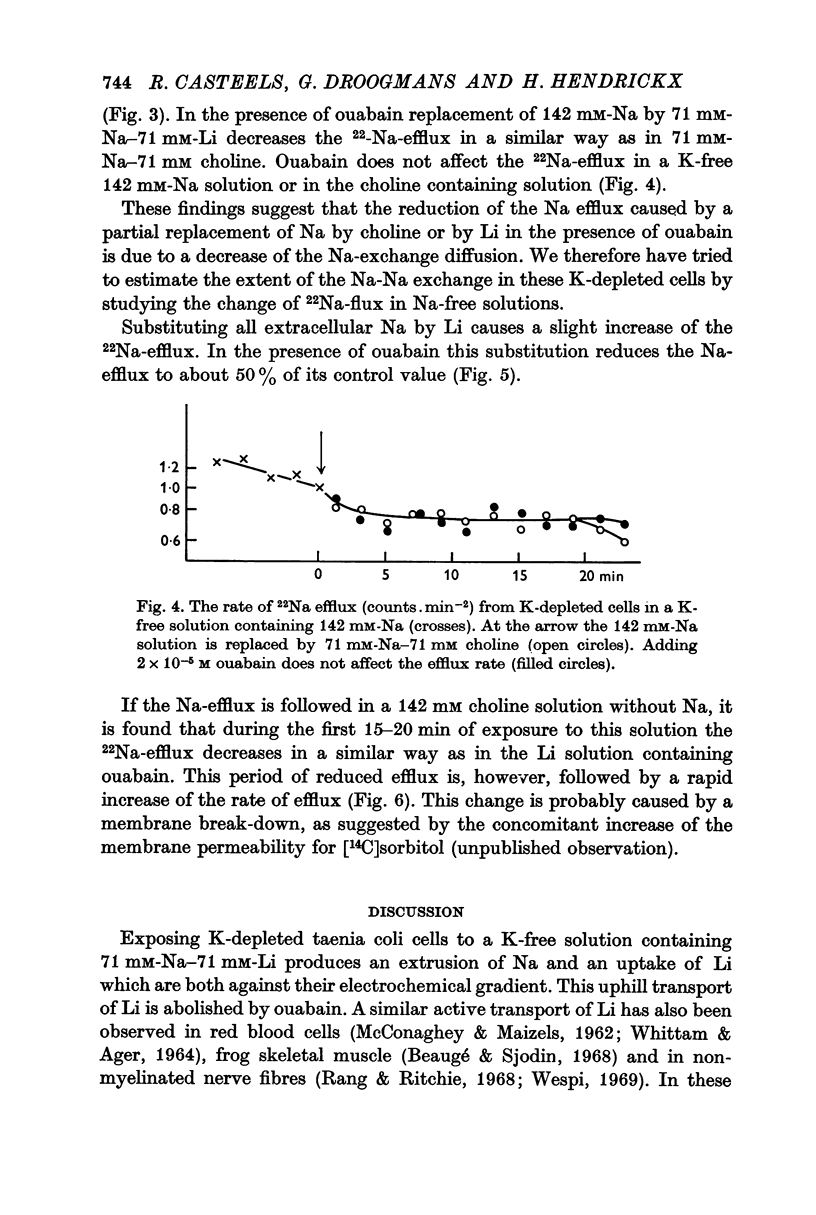
6. In Na-enriched tissues half of the Na efflux is due to a ouabain insensitive Na-exchange diffusion. If Li is used as a Na substitute, the Na-Li exchange compensates for the diminution of the Na-exchange diffusion unless ouabain is added.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Connelly C. M. Some properties of the external activation site of the sodium pump in crab nerve. J Physiol. 1966 Jul;185(2):270–297. doi: 10.1113/jphysiol.1966.sp007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Sjodin R. A. The dual effect of lithium ions on sodium efflux in skeletal muscle. J Gen Physiol. 1968 Sep;52(3):408–423. doi: 10.1085/jgp.52.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Electrogenic sodium pump in smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Sep;217(2):297–313. doi: 10.1113/jphysiol.1971.sp009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Membrane potential of smooth muscle cells in K-free solution. J Physiol. 1971 Sep;217(2):281–295. doi: 10.1113/jphysiol.1971.sp009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Taylor J. W., Waggoner D. M. Fractionation of sodium effux in frog sartorius muscles by strophanthidin and removal of external sodium. J Gen Physiol. 1970 Mar;55(3):401–425. doi: 10.1085/jgp.55.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels M. Effect of sodium content on sodium efflux from human red cells suspended in sodium-free media containing potassium, rubidium, caesium or lithium chloride. J Physiol. 1968 Apr;195(3):657–679. doi: 10.1113/jphysiol.1968.sp008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaghey P. D., Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962 Aug;162(3):485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- Priestland R. N., Whittam R. The influence of external sodium ions on the sodium pump in erythrocytes. Biochem J. 1968 Sep;109(3):369–374. doi: 10.1042/bj1090369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A. The kinetics of sodium extrusion in striated muscle as functions of the external sodium and potassium ion concentrations. J Gen Physiol. 1971 Feb;57(2):164–187. doi: 10.1085/jgp.57.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. Transport of ions across cellular membranes. Physiol Rev. 1949 Apr;29(2):127–155. doi: 10.1152/physrev.1949.29.2.127. [DOI] [PubMed] [Google Scholar]
- Wespi H. H. Active transport and passive fluxes of K, Na, and Li in mammalian non-myelinated nerve fibres. Pflugers Arch. 1969;306(3):262–280. doi: 10.1007/BF00592437. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]


