Abstract
1. Increment thresholds were measured on the albino rat retina using the e.r.g. as an indicator of visual sensitivity.
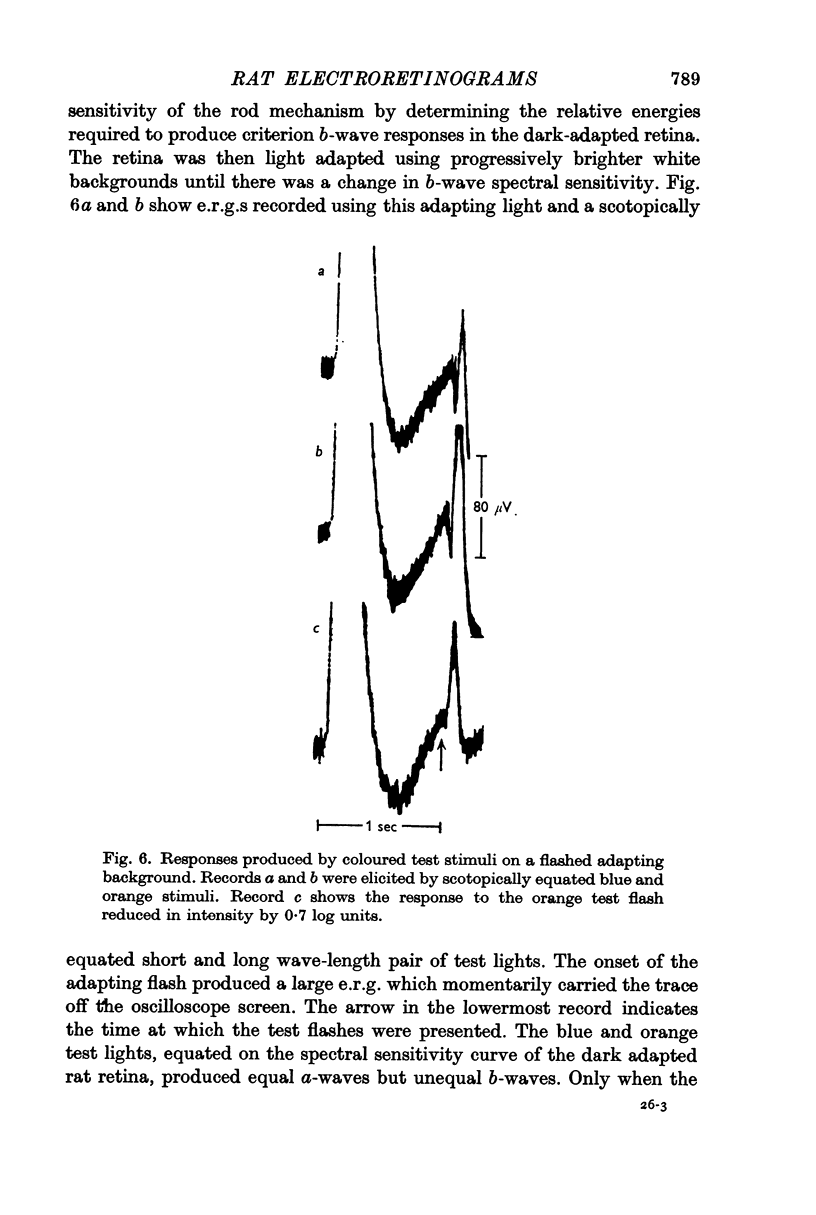
2. Light adaptation produces a change in b-wave spectral sensitivity independently of whether brief or steady adapting backgrounds are used. This shows that the changed spectral sensitivity is not directly associated with the bleaching of visual pigment.
3. Light adaptation does not appear to alter the spectral sensitivity of the ordinary a-wave. Consequently, after light adaptation a- and b-waves have different spectral sensitivities.
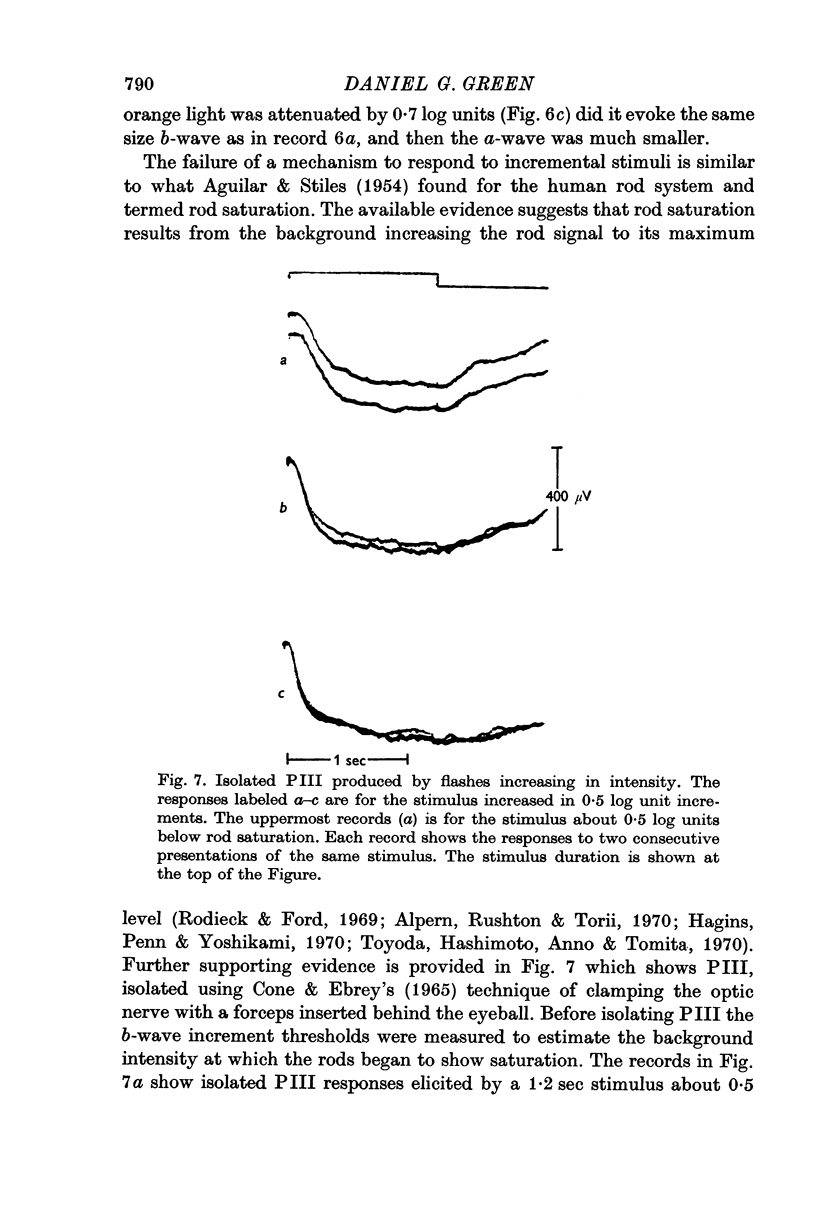
4. B-wave increments determined with a blue test stimulus on a red adaptive background show rod saturation. Background levels which saturate the rods cause the a-wave mechanism to cease responding to incremental stimuli.
5. A background intense enough to saturate the rods evokes a maximum a-wave response. If a light is sufficiently bright to saturate the rods then the isolated a-wave fails to signal the termination of the stimulus.
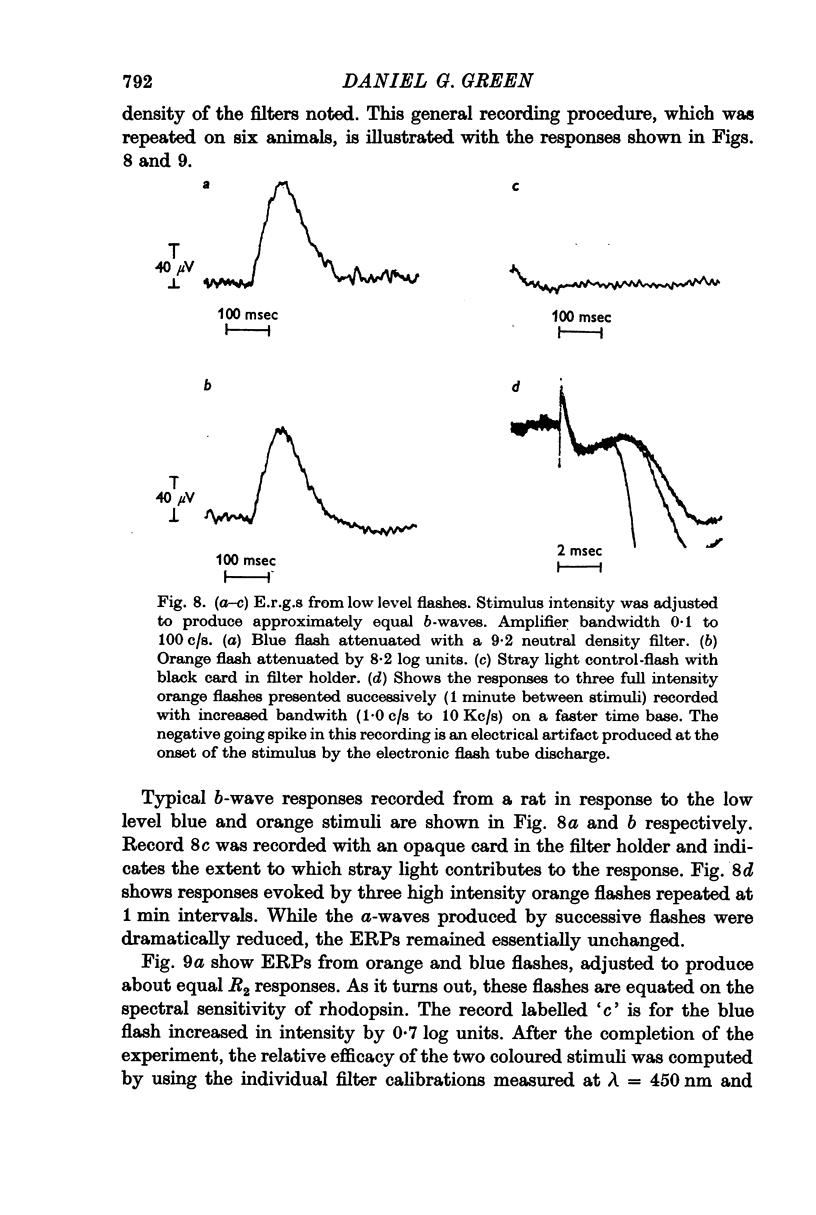
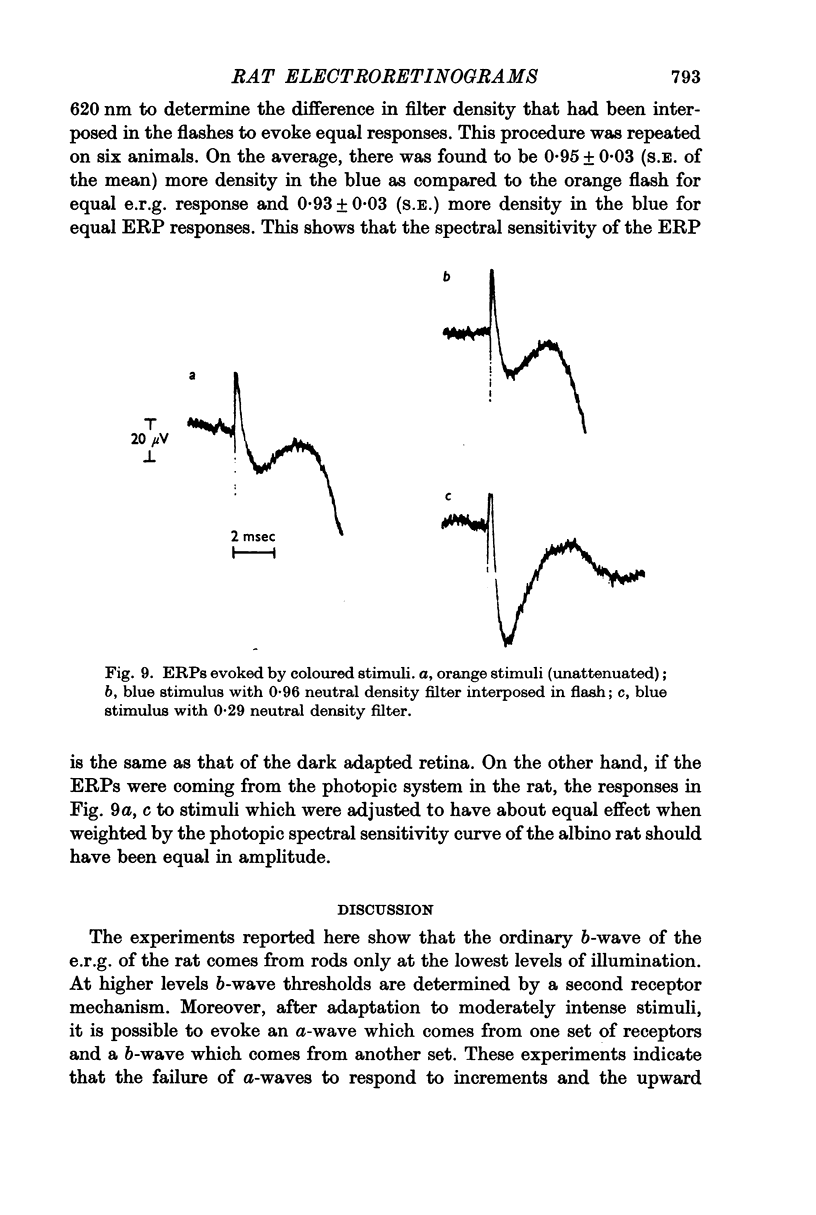
6. Scotopically equated blue and orange stimuli produce equal early receptor potentials.
Full text
PDF


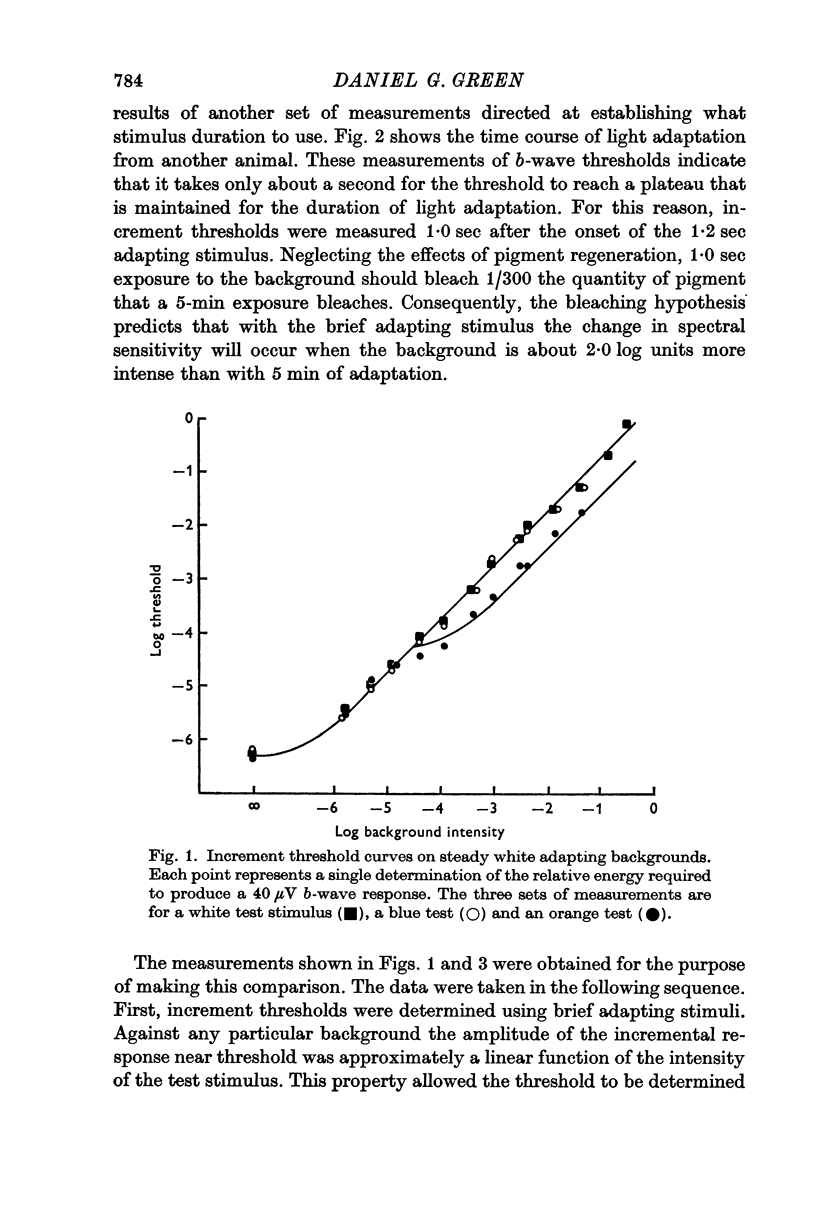
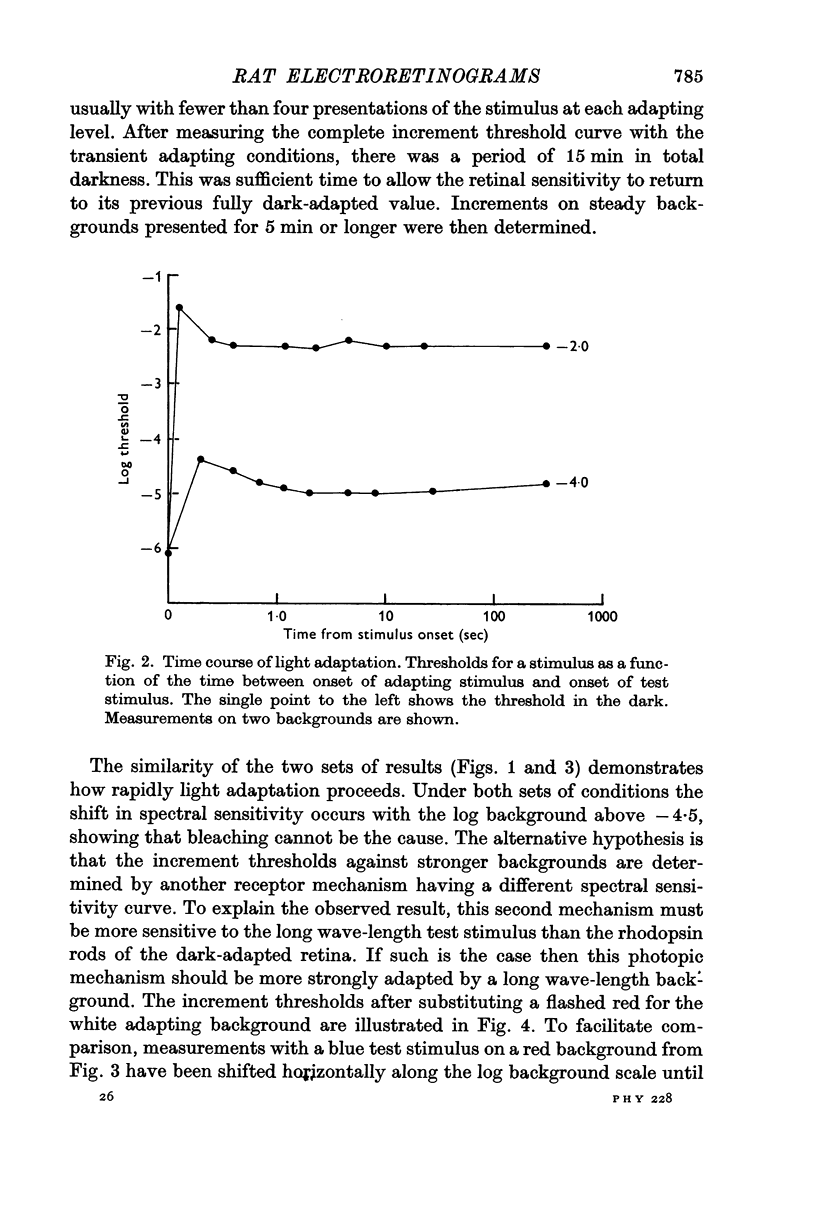
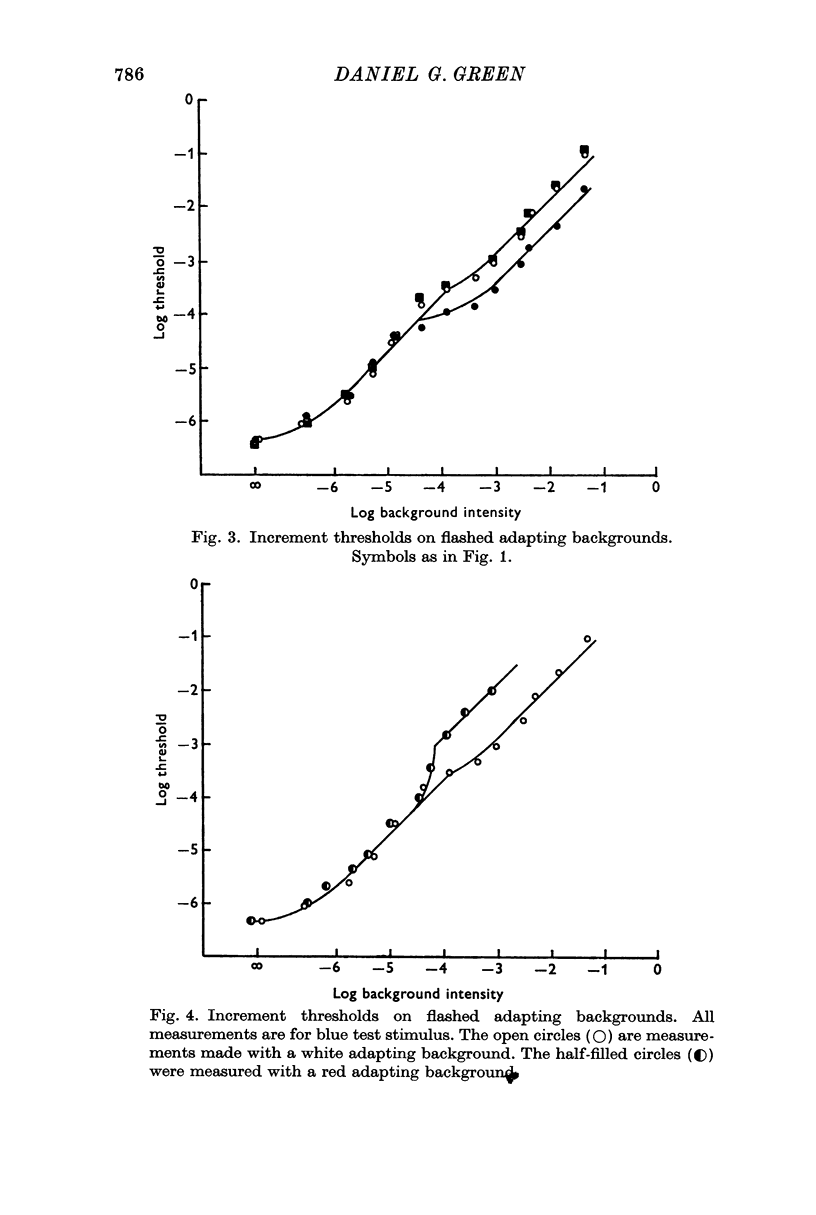
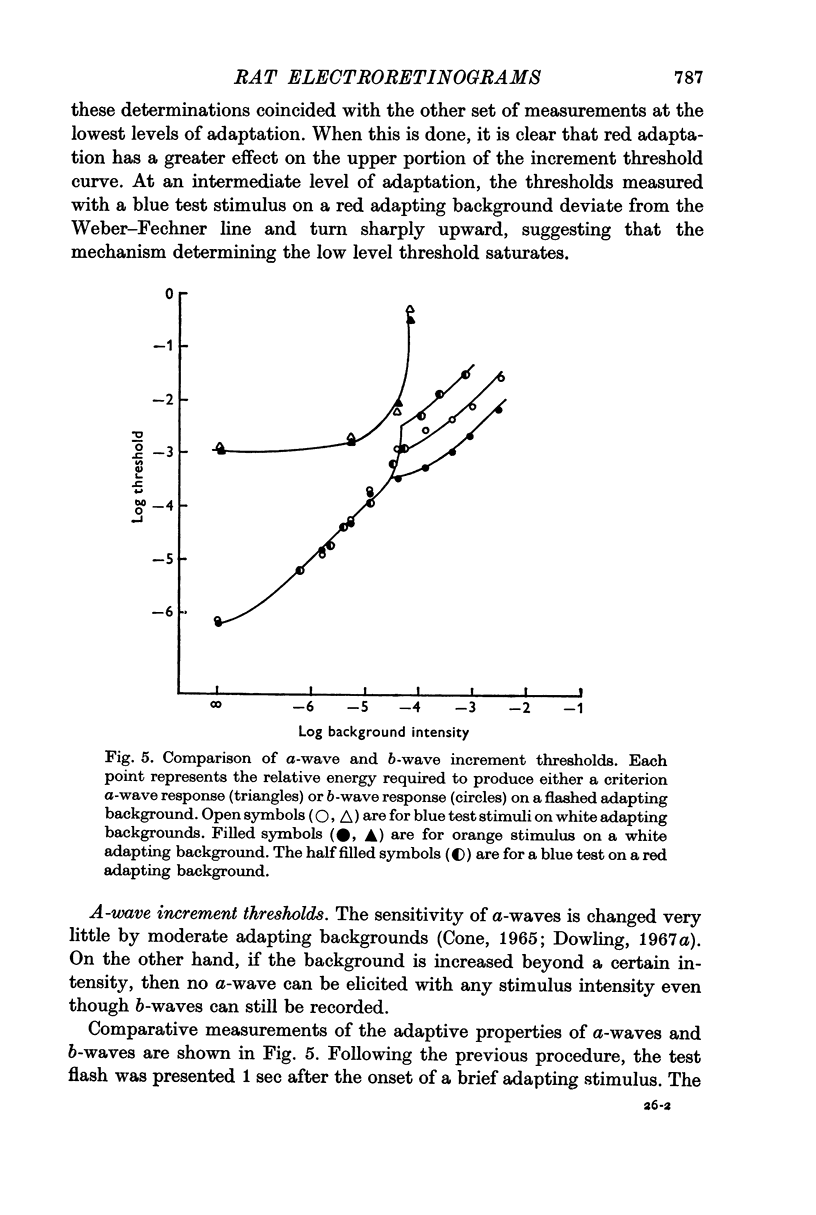






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M., Rushton W. A., Torii S. Signals from cones. J Physiol. 1970 Apr;207(2):463–475. doi: 10.1113/jphysiol.1970.sp009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN K. T., MURAKAMI M. A NEW RECEPTOR POTENTIAL OF THE MONKEY RETINA WITH NO DETECTABLE LATENCY. Nature. 1964 Feb 8;201:626–628. doi: 10.1038/201626a0. [DOI] [PubMed] [Google Scholar]
- BROWN K. T., WIESEL T. N. Analysis of the intraretinal electroretinogram in the intact cat eye. J Physiol. 1961 Sep;158:229–256. doi: 10.1113/jphysiol.1961.sp006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. T., Murakami M. Delayed decay of the late receptor potential of monkey cones as a function of stimulus intensity. Vision Res. 1967 Mar;7(3):179–189. doi: 10.1016/0042-6989(67)90082-x. [DOI] [PubMed] [Google Scholar]
- CONE R. A. EARLY RECEPTOR POTENTIAL OF THE VERTEBRATE RETINA. Nature. 1964 Nov 21;204:736–739. doi: 10.1038/204736a0. [DOI] [PubMed] [Google Scholar]
- Cone R. A., Cobbs W. H., 3rd Rhodopsin cycle in the living eye of the rat. Nature. 1969 Mar 1;221(5183):820–822. doi: 10.1038/221820a0. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Early receptor potential: photoreversible charge displacement in rhodopsin. Science. 1967 Mar 3;155(3766):1128–1131. doi: 10.1126/science.155.3766.1128. [DOI] [PubMed] [Google Scholar]
- Cone R. A., Ebrey T. G. Functional independence of the two major components of the rod electroretinogram. Nature. 1965 May 29;206(987):913–915. doi: 10.1038/206913a0. [DOI] [PubMed] [Google Scholar]
- Cone R. A. The early receptor potential of the vertebrate eye. Cold Spring Harb Symp Quant Biol. 1965;30:483–491. doi: 10.1101/sqb.1965.030.01.046. [DOI] [PubMed] [Google Scholar]
- DODT E., ECHTE K. Dark and light adaptation in pigmented and white rat as measured by electroretinogram threshold. J Neurophysiol. 1961 Jul;24:427–445. doi: 10.1152/jn.1961.24.4.427. [DOI] [PubMed] [Google Scholar]
- DOWLING J. E. Chemistry of visual adaptation in the rat. Nature. 1960 Oct 8;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- DOWLING J. E. NEURAL AND PHOTOCHEMICAL MECHANISMS OF VISUAL ADAPTATION IN THE RAT. J Gen Physiol. 1963 Jul;46:1287–1301. doi: 10.1085/jgp.46.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Rod saturation in the cat. Vision Res. 1971 Nov;11(11):1361–1364. doi: 10.1016/0042-6989(71)90020-4. [DOI] [PubMed] [Google Scholar]
- Dowling J. E. The site of visual adaptation. Science. 1967 Jan 20;155(3760):273–279. doi: 10.1126/science.155.3760.273. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Wald G. VITAMIN A DEFICIENCY AND NIGHT BLINDNESS. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Cone R. A. Melanin, a possible pigment for the photostable electrical responses of the eye. Nature. 1967 Jan 28;213(5074):360–362. doi: 10.1038/213360a0. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G. The thermal decay of the intermediates of rhodopsin in situ. Vision Res. 1968 Aug;8(8):965–982. doi: 10.1016/0042-6989(68)90071-0. [DOI] [PubMed] [Google Scholar]
- Goldstein E. B. Contribution of cones to the early receptor potential in the rhesus monkey. Nature. 1969 Jun 28;222(5200):1273–1274. doi: 10.1038/2221273a0. [DOI] [PubMed] [Google Scholar]
- Goldstein E. B. Contribution of cones to the early receptor potential in the rhesus monkey. Nature. 1969 Jun 28;222(5200):1273–1274. doi: 10.1038/2221273a0. [DOI] [PubMed] [Google Scholar]
- Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol. 1933 Feb 8;77(3):207–239. doi: 10.1113/jphysiol.1933.sp002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Rüppel H. Fast photoelectric effects and the properties of vertebrate photoreceptors as electric cables. Fed Proc. 1971 Jan-Feb;30(1):64–68. [PubMed] [Google Scholar]
- Miller R. F., Dowling J. E. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970 May;33(3):323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kaneko A. Subcomponents of P3 in cold-blooded vertebrate retinae. Nature. 1966 Apr 2;210(5031):103–104. doi: 10.1038/210103a0. [DOI] [PubMed] [Google Scholar]
- Pautler E. L., Murakami M., Nosaki H. Differentiation of P 3 subcomponents in isolated mammalian retinas. Vision Res. 1968 Apr;8(4):489–491. doi: 10.1016/0042-6989(68)90118-1. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. Rhodopsin measurement and dark-adaptation in a subject deficient in cone vision. J Physiol. 1961 Apr;156:193–205. doi: 10.1113/jphysiol.1961.sp006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Ford R. W. The cat local electroretinogram to incremental stimuli. Vision Res. 1969 Jan;9(1):1–24. doi: 10.1016/0042-6989(69)90028-5. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Winkler B. S. The electroretinogram of the isolated rat retina. Vision Res. 1972 Jun;12(6):1183–1198. doi: 10.1016/0042-6989(72)90106-x. [DOI] [PubMed] [Google Scholar]



