Abstract
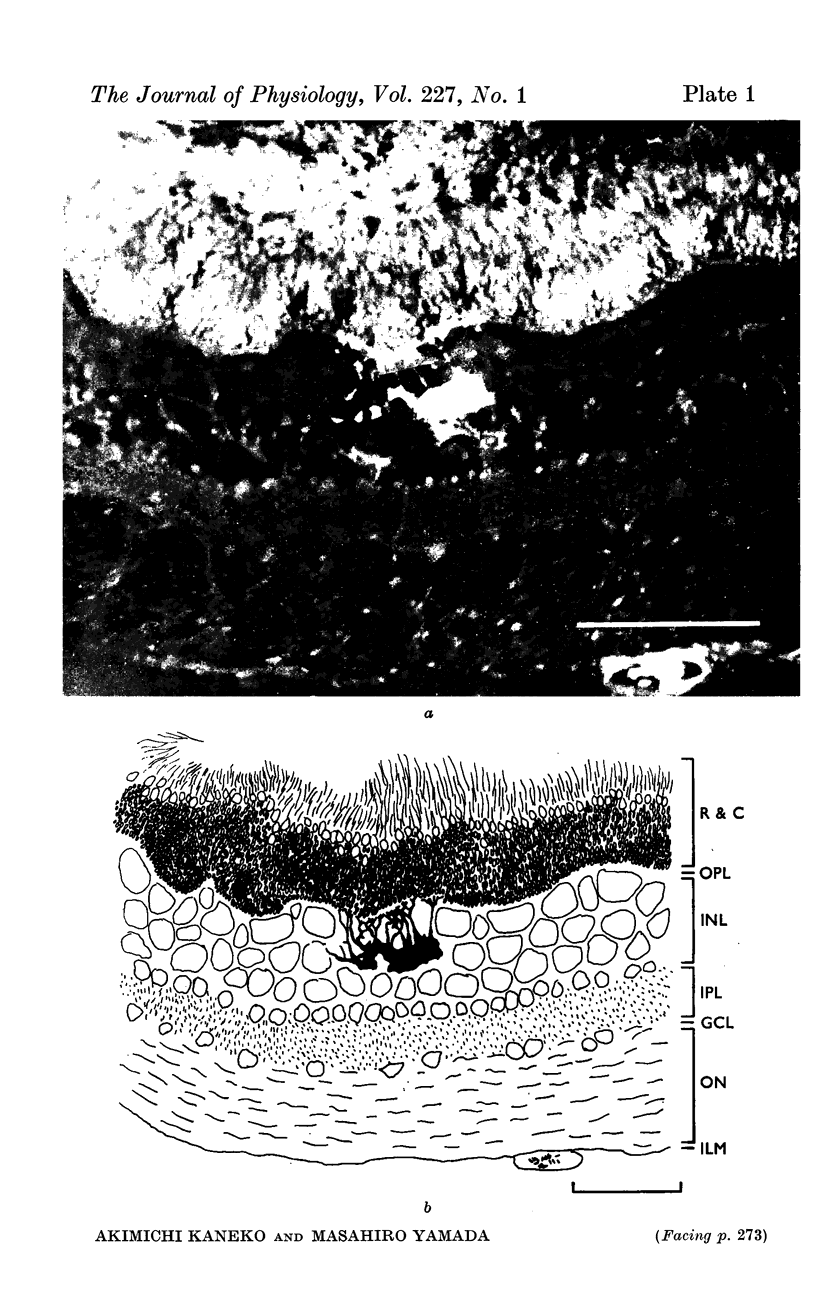
1. S-potentials were recorded in the dark-adapted carp retina. After recording, Procion Yellow was injected from the recording electrode to identify histologically the cell recorded.
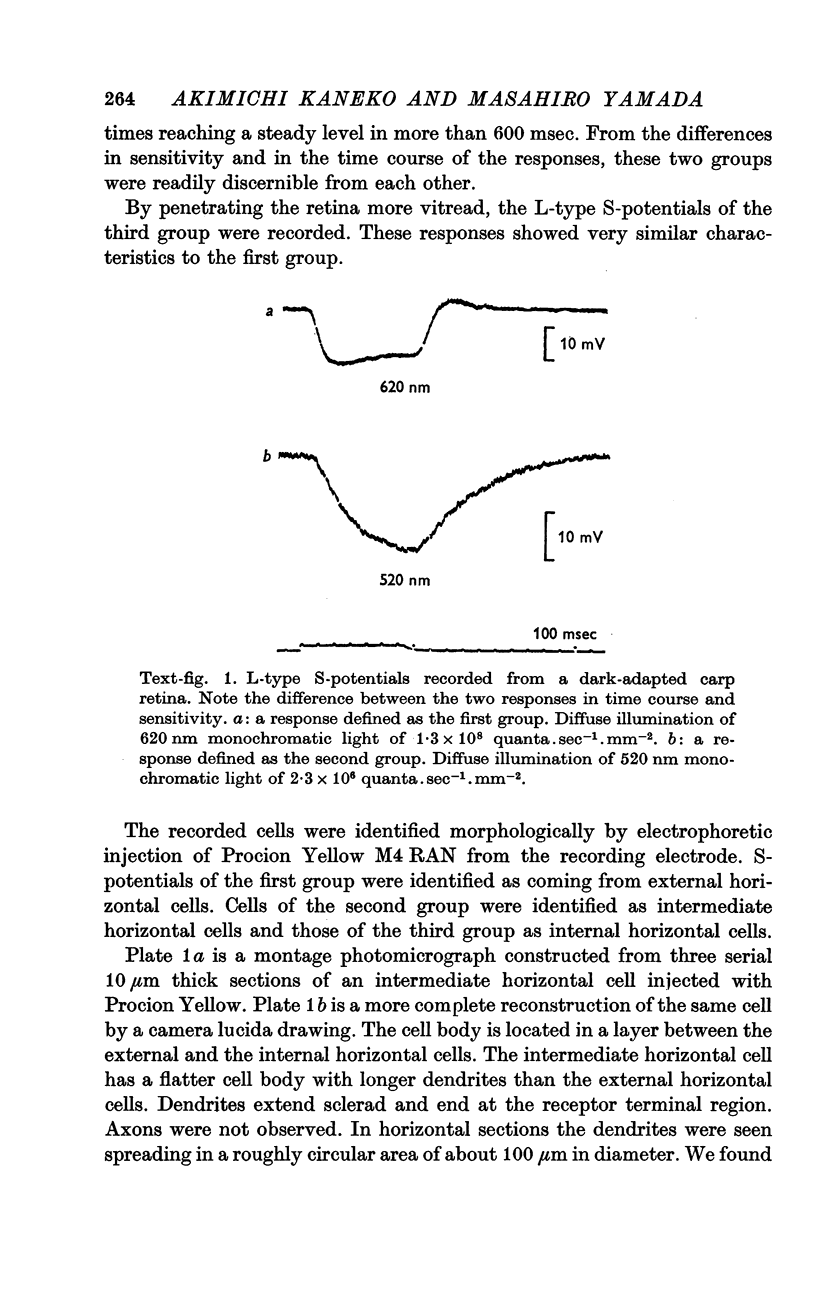
2. Both L- and C-type S-potentials were found. L-type S-potentials were further classified into two groups. The first group was characterized by a high threshold, fast rise and fall times and a spectral sensitivity peak of about 620 nm. The second group showed a low threshold, slow response time course and a spectral sensitivity peak of about 520 nm.
3. Cells of the first group were identified as external and internal horizontal cells. Cells of the second group were identified as intermediate horizontal cells.
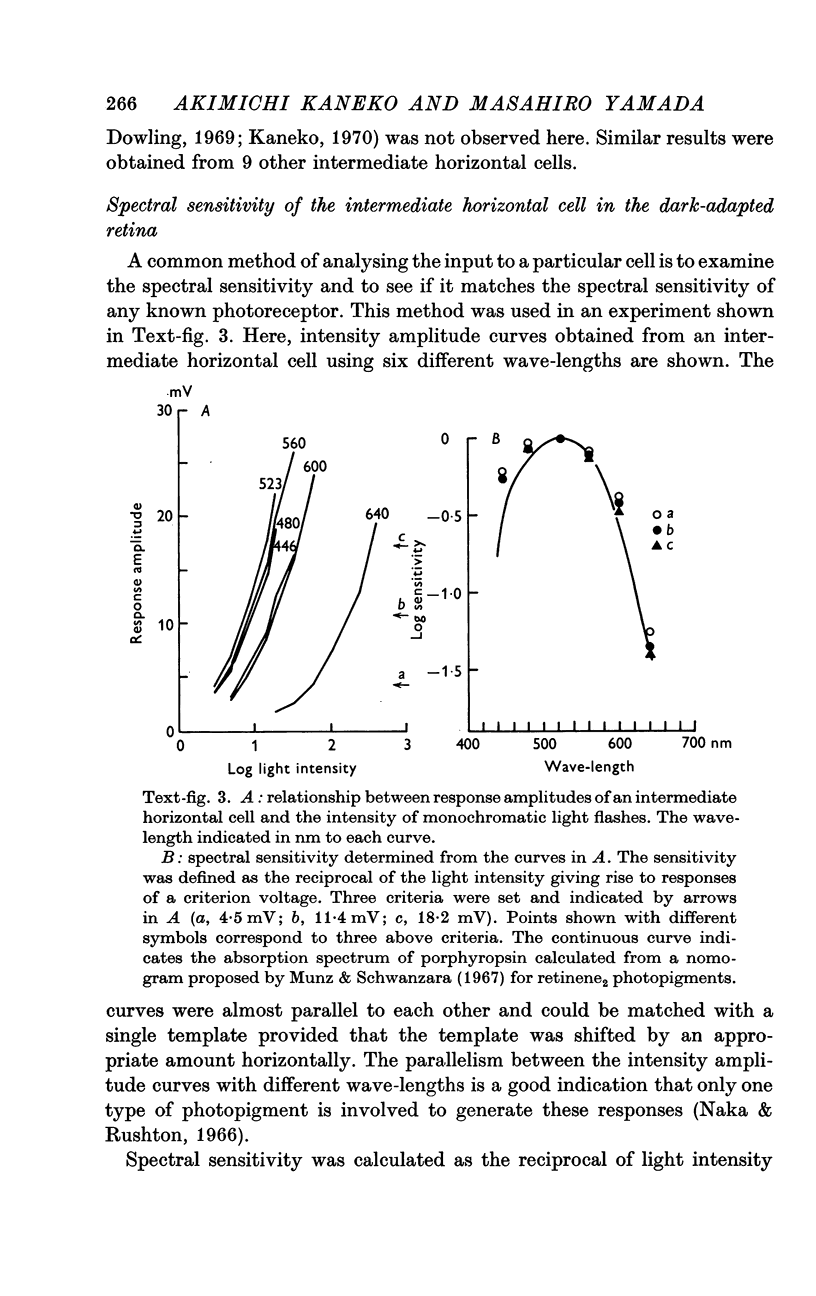
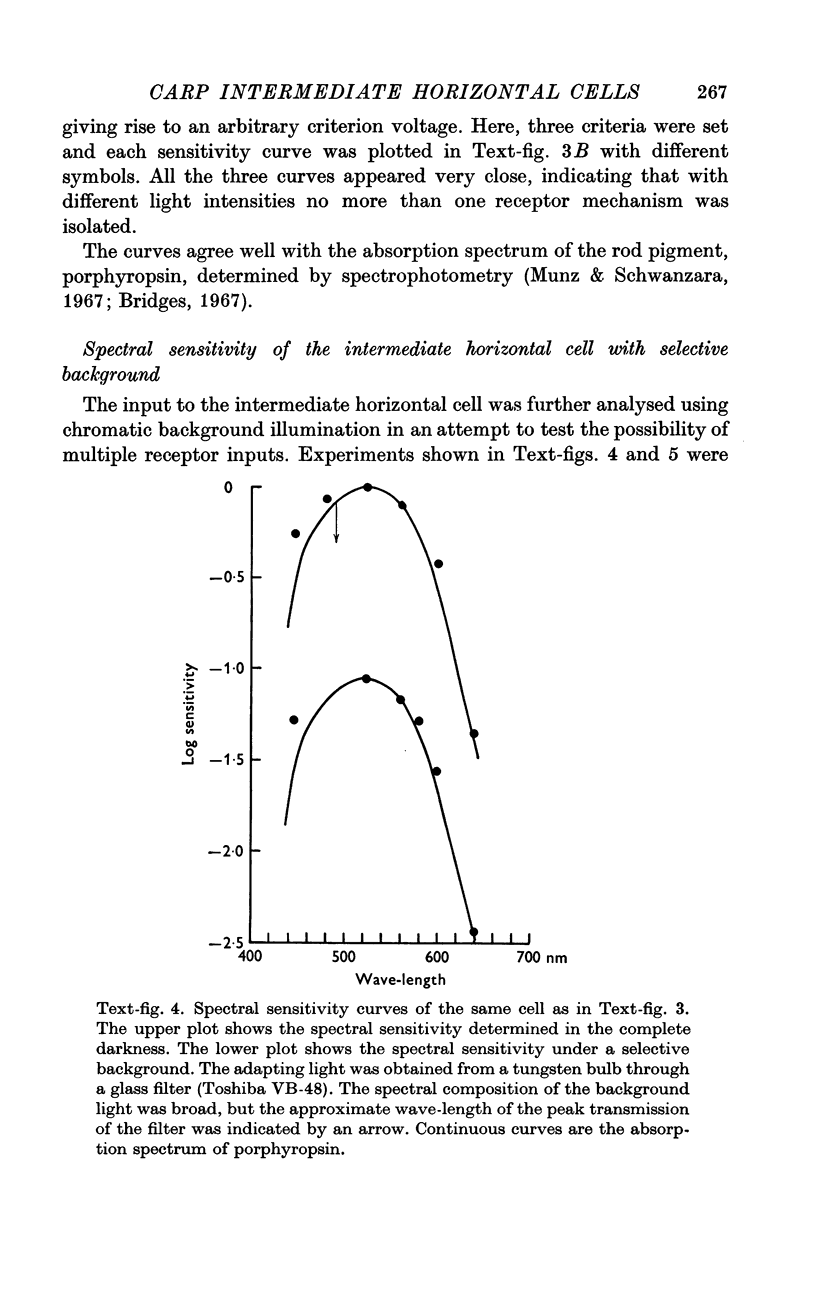
4. In the dark-adapted retina, the spectral sensitivity of the intermediate horizontal cell showed a good agreement with the absorption spectrum of the rod pigment, porphyropsin.
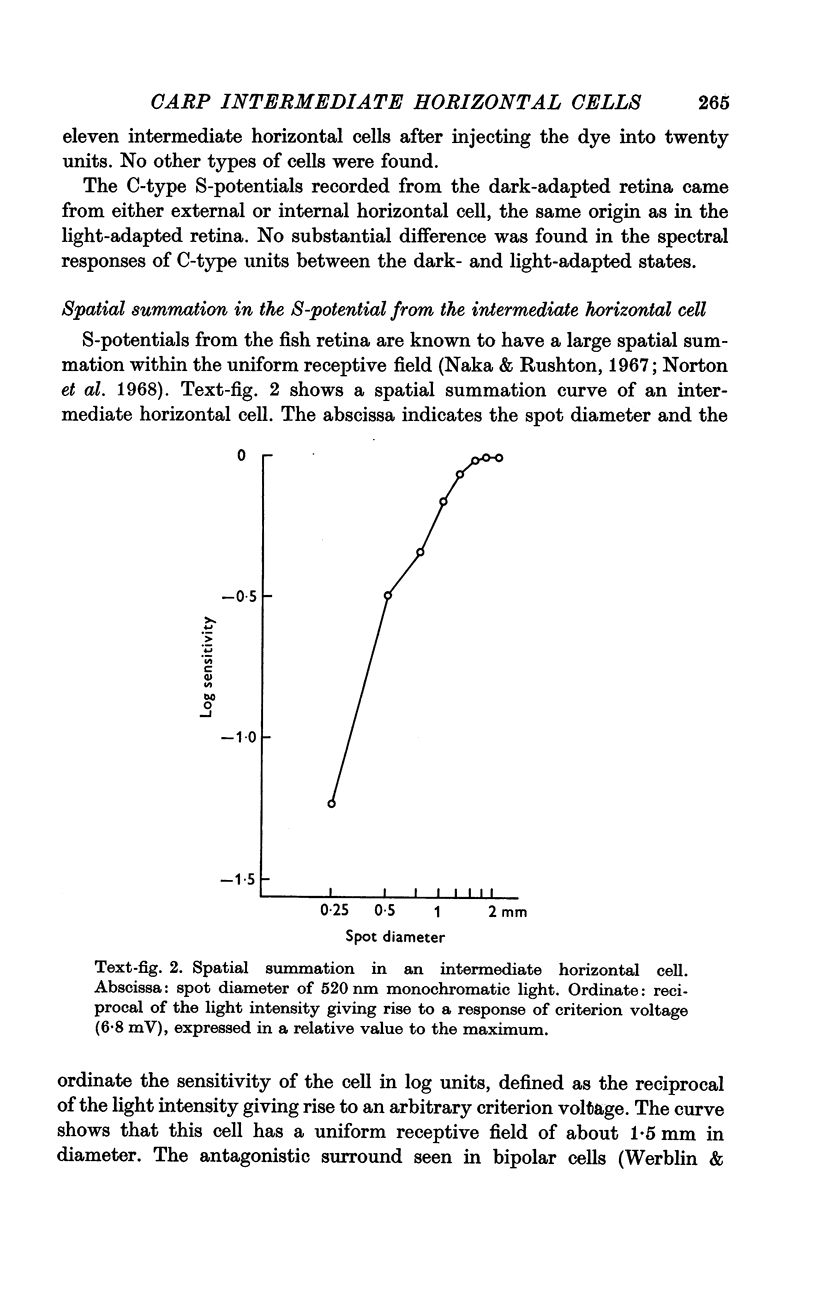
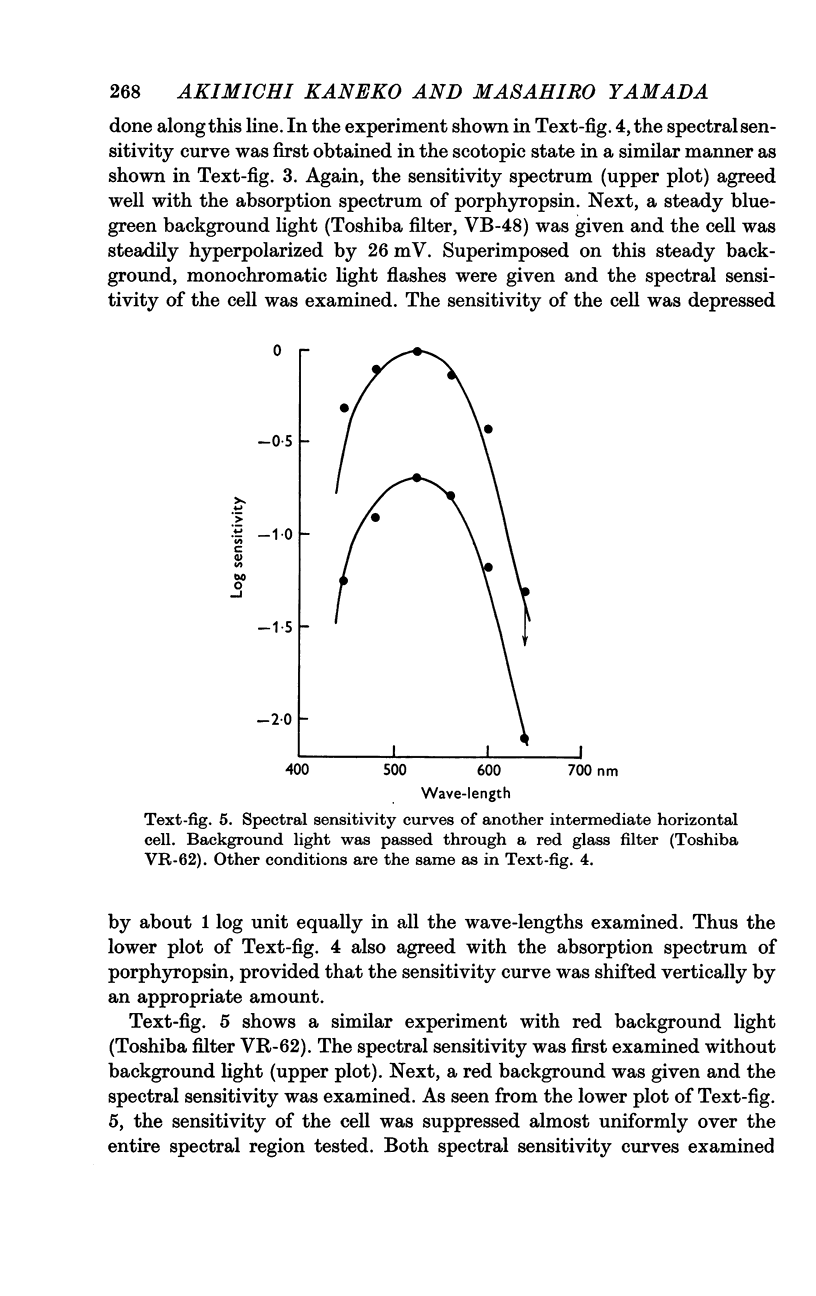
5. Neither a chromatic background nor white background illumination of moderate intensity (1 cd.m-2) shifted the spectral sensitivity of the intermediate horizontal cell.
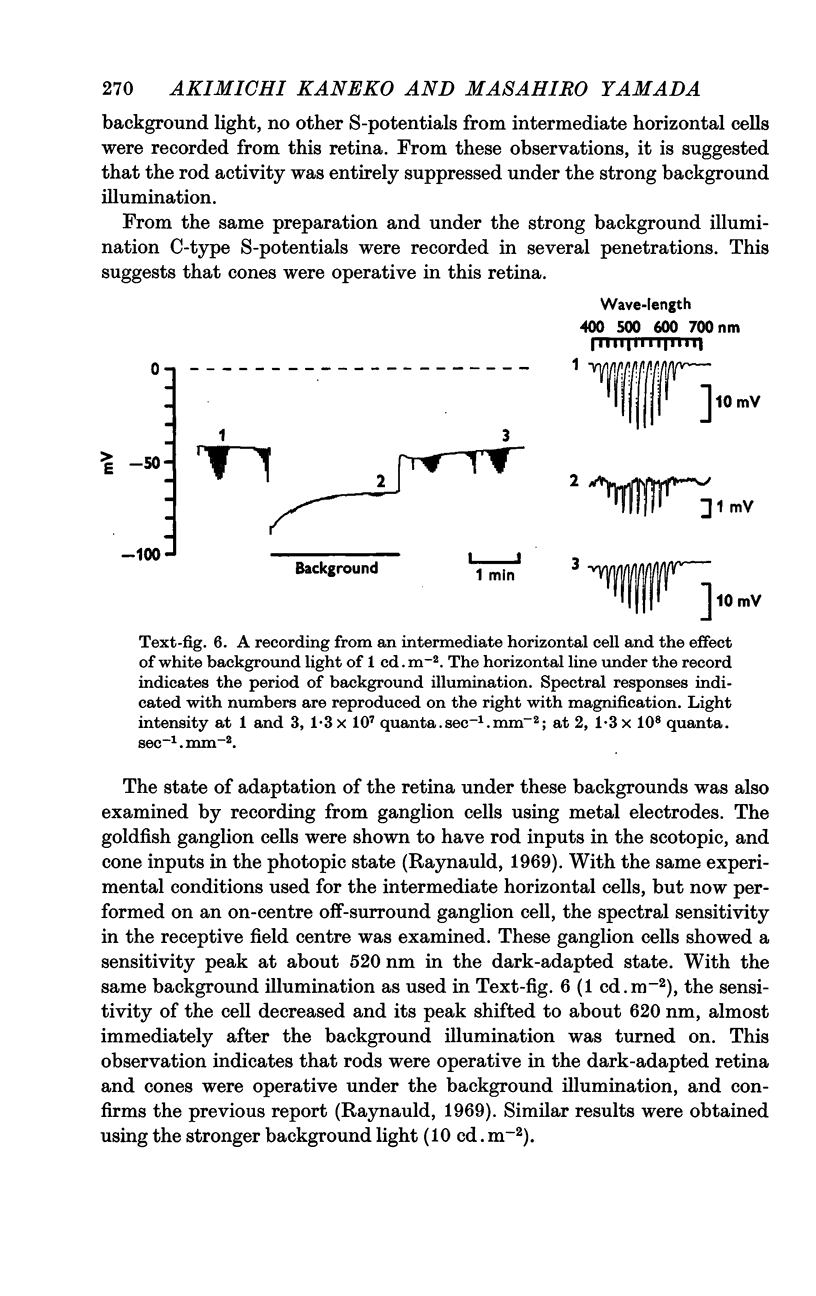
6. Under a strong background (10 cd.m-2), the intermediate horizontal cell was entirely suppressed. Under the same conditions the external horizontal cell was still capable of responding.
7. From these observations it is concluded that the intermediate horizontal cell receives input from rods only.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges C. D. Spectroscopic properties of porphyropsins. Vision Res. 1967 May;7(5):349–369. doi: 10.1016/0042-6989(67)90044-2. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- MARKS W. B. VISUAL PIGMENTS OF SINGLE GOLDFISH CONES. J Physiol. 1965 May;178:14–32. doi: 10.1113/jphysiol.1965.sp007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz F. W., Schwanzara S. A. A nomogram for retinene-2-based visual pigments. Vision Res. 1967 Mar;7(3):111–120. doi: 10.1016/0042-6989(67)90078-8. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Schmidt R. Identification of horizontal cells as S-potential generators in the cat retina by intracellular dye injection. Vision Res. 1970 Sep;10(9):817–820. doi: 10.1016/0042-6989(70)90160-4. [DOI] [PubMed] [Google Scholar]
- Stell W. K. The structure and relationships of horizontal cells and photoreceptor-bipolar synaptic complexes in goldfish retina. Am J Anat. 1967 Sep;121(2):401–423. doi: 10.1002/aja.1001210213. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A., Murakami M., Pautler E. L. Spectral response curves of single cones in the carp. Vision Res. 1967 Jul;7(7):519–531. doi: 10.1016/0042-6989(67)90061-2. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. A comparison of ganglion cell and S-potential response properties in carp retina. J Neurophysiol. 1967 May;30(3):546–561. doi: 10.1152/jn.1967.30.3.546. [DOI] [PubMed] [Google Scholar]