Abstract
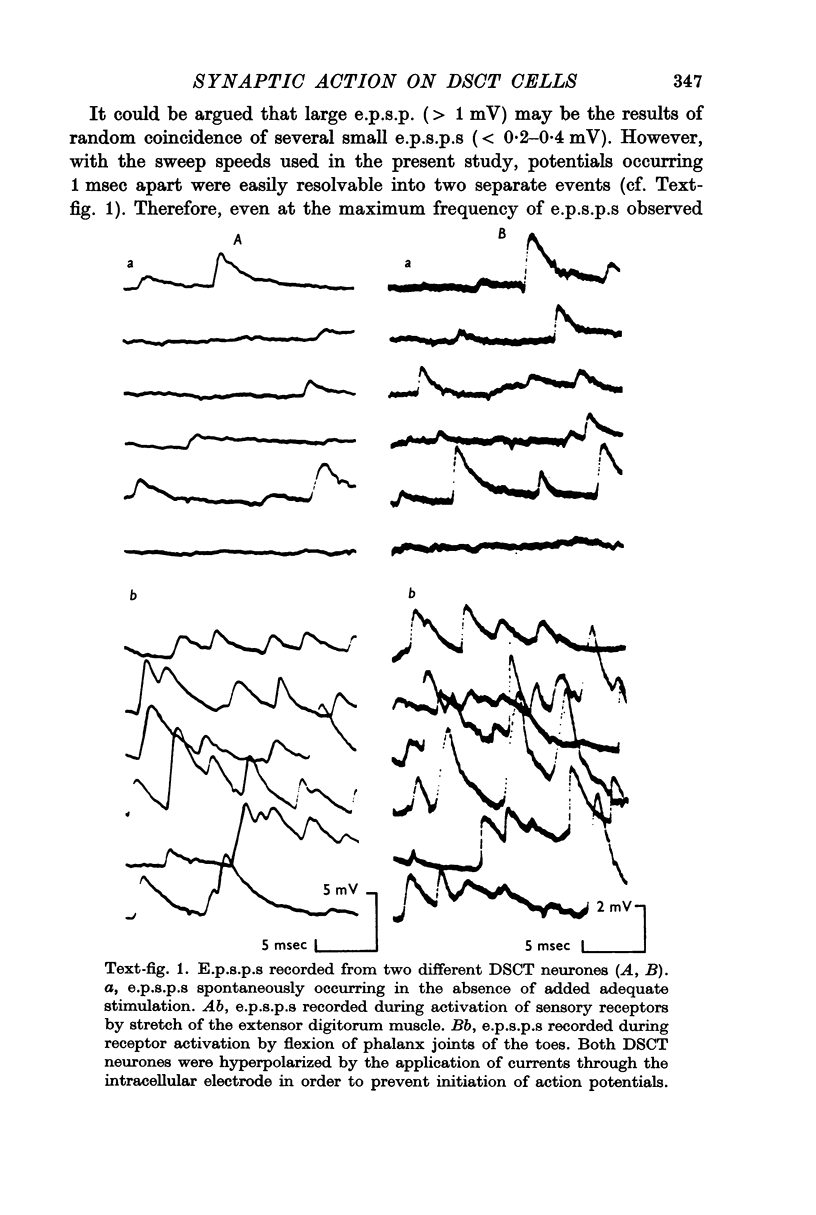
1. Excitatory post-synaptic potentials (e.p.s.p.s) were recorded intracellularly from Clarke's column neurones (DSCT neurones) of the cat in response to adequate stimuli applied to a variety of sensory receptors.
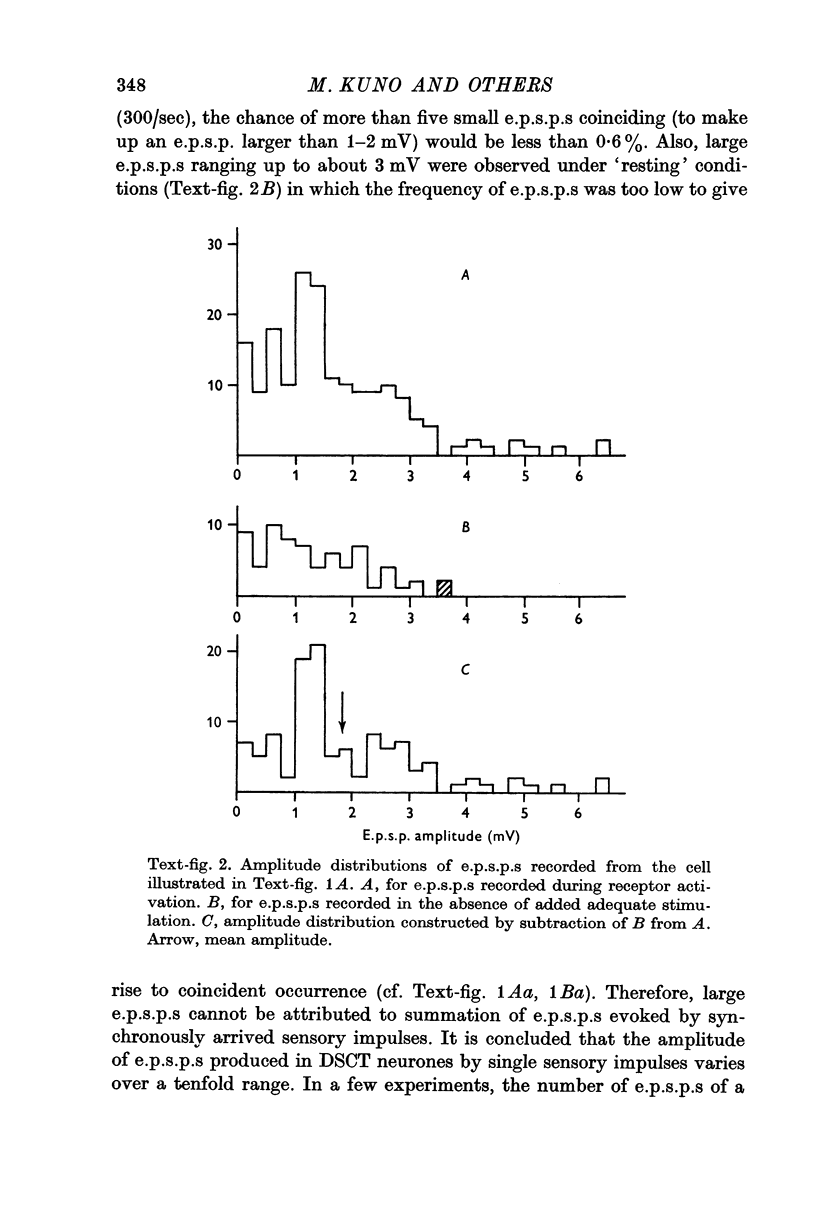
2. The amplitude of e.p.s.p.s so produced varied from less than 0·2 mV to more than 2-3 mV. The amplitude distribution of e.p.s.p.s suggested that the mean number of `quanta' of transmitter released by one impulse varied widely from one fibre to another arising from a given type of sensory receptor.
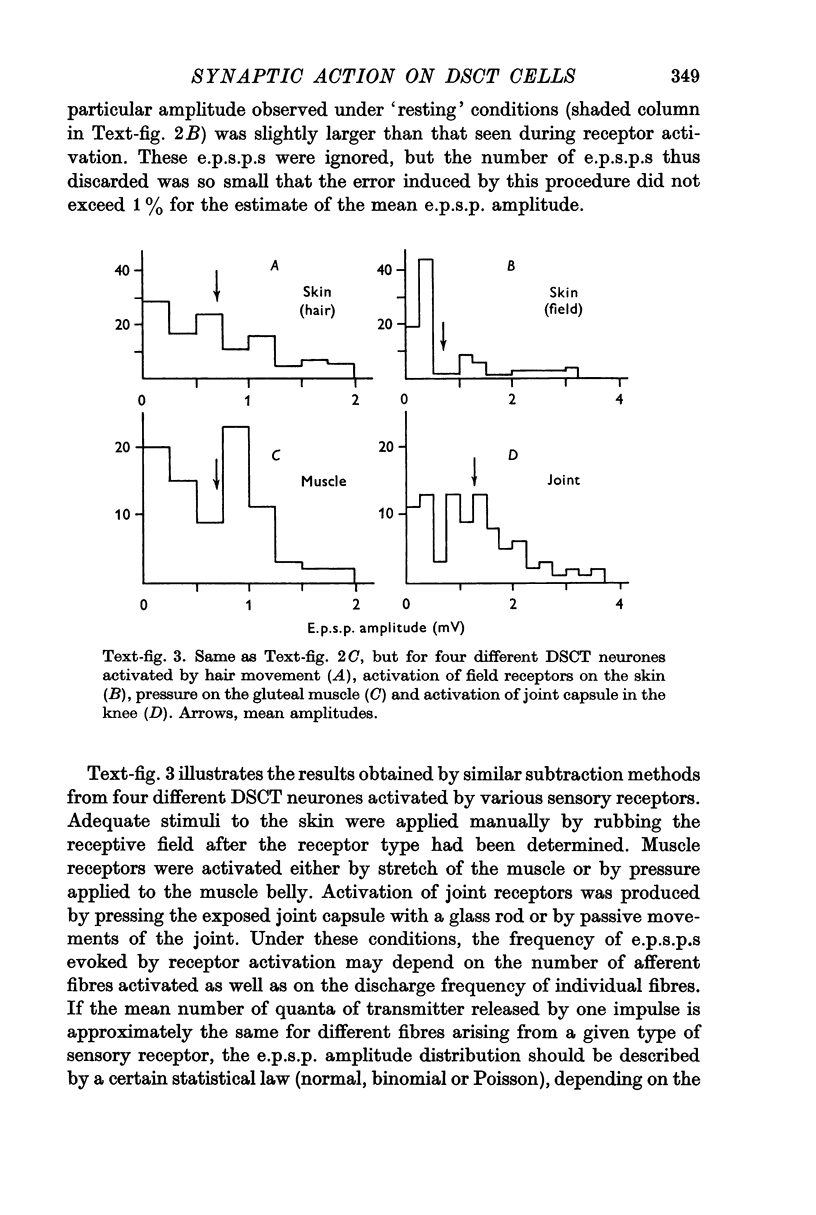
3. The average amplitude of e.p.s.p.s evoked by single afferent impulses was significantly smaller for cutaneous inputs than for muscle or joint inputs. However, synaptic action on DSCT neurones produced by different sensory inputs was equally greater, on the average, than that on spinal motoneurones.
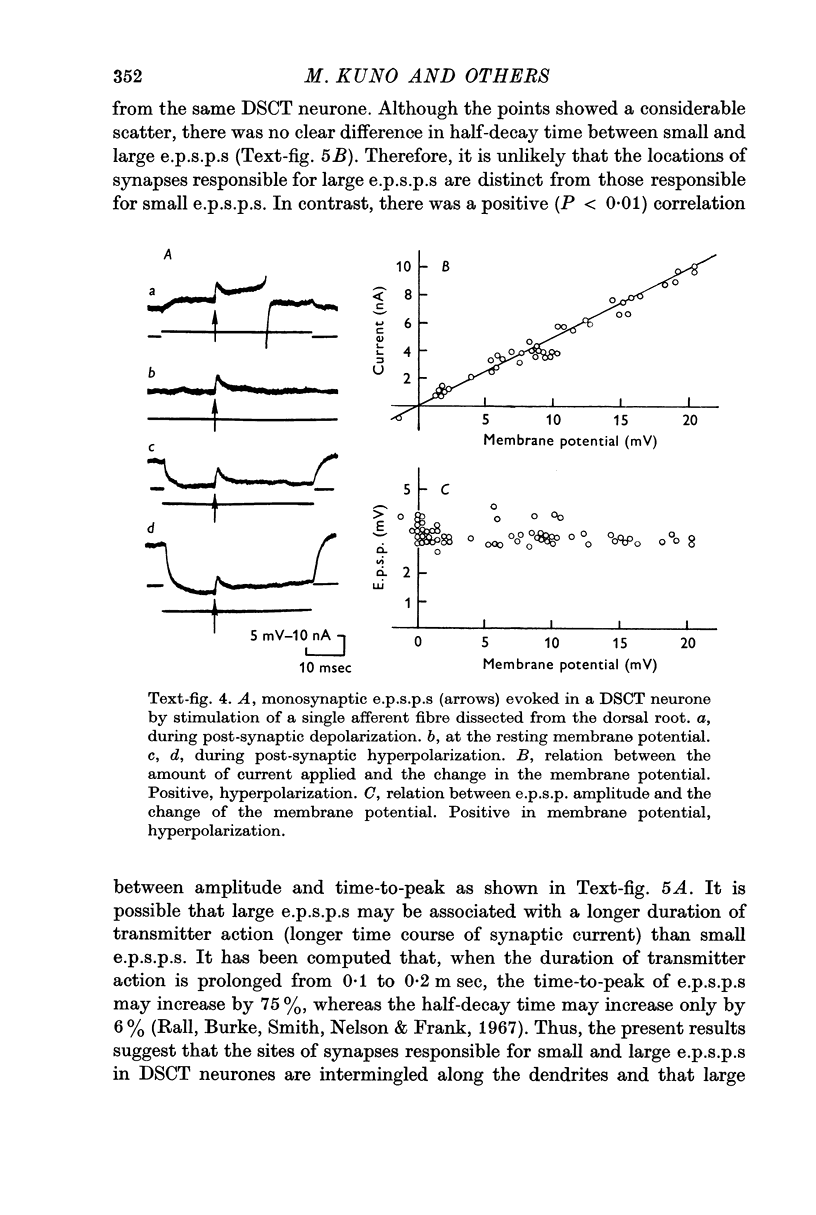
4. Both small and large e.p.s.p.s in DSCT neurones failed to increase in amplitude during post-synaptic hyperpolarization applied through the cell body. This failure could not be attributed to possible anomalous rectification in the post-synaptic membrane.
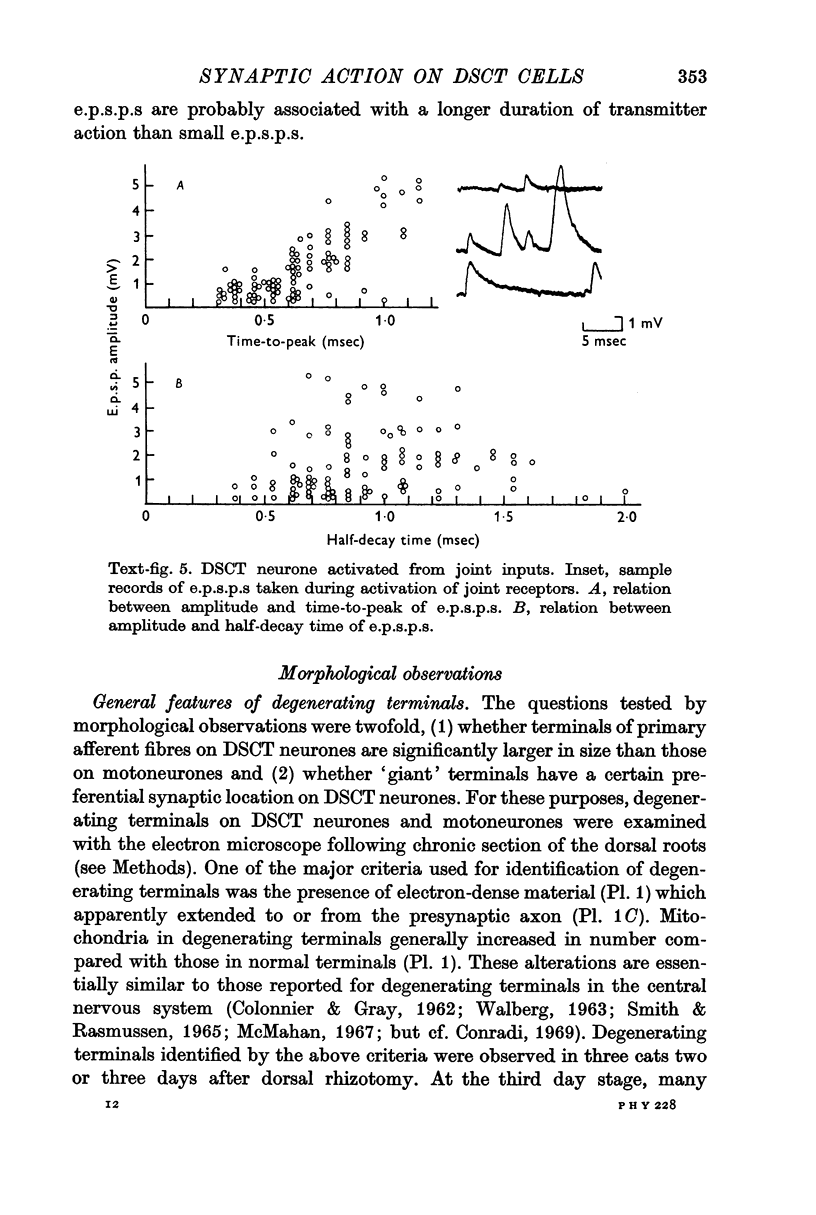
5. Small and large e.p.s.p.s were comparable in half-decay time, but there was a positive correlation between amplitude and time-to-peak of e.p.s.p.s. It is suggested that the locations of synapses responsible for small and large e.p.s.p.s are intermingled on the dendrites and that large e.p.s.p.s are associated with a longer duration of transmitter action than small e.p.s.p.s.
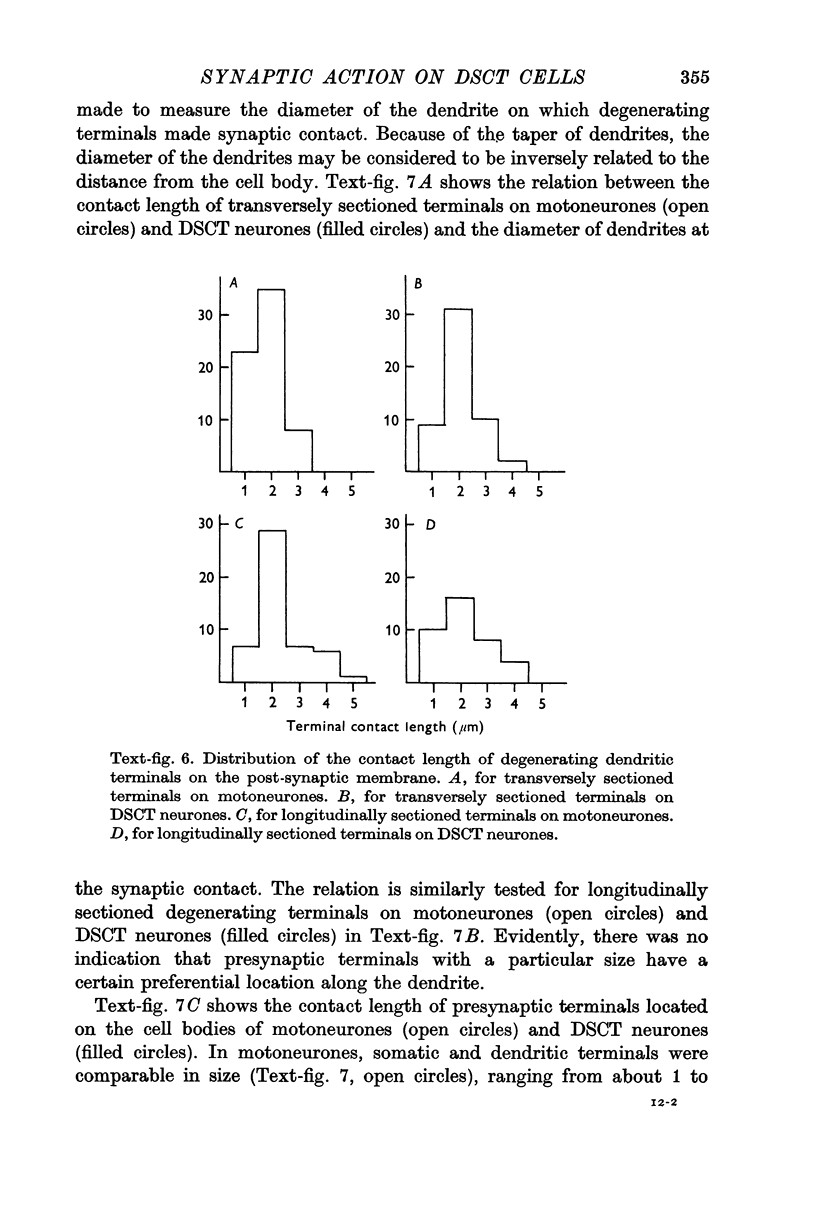
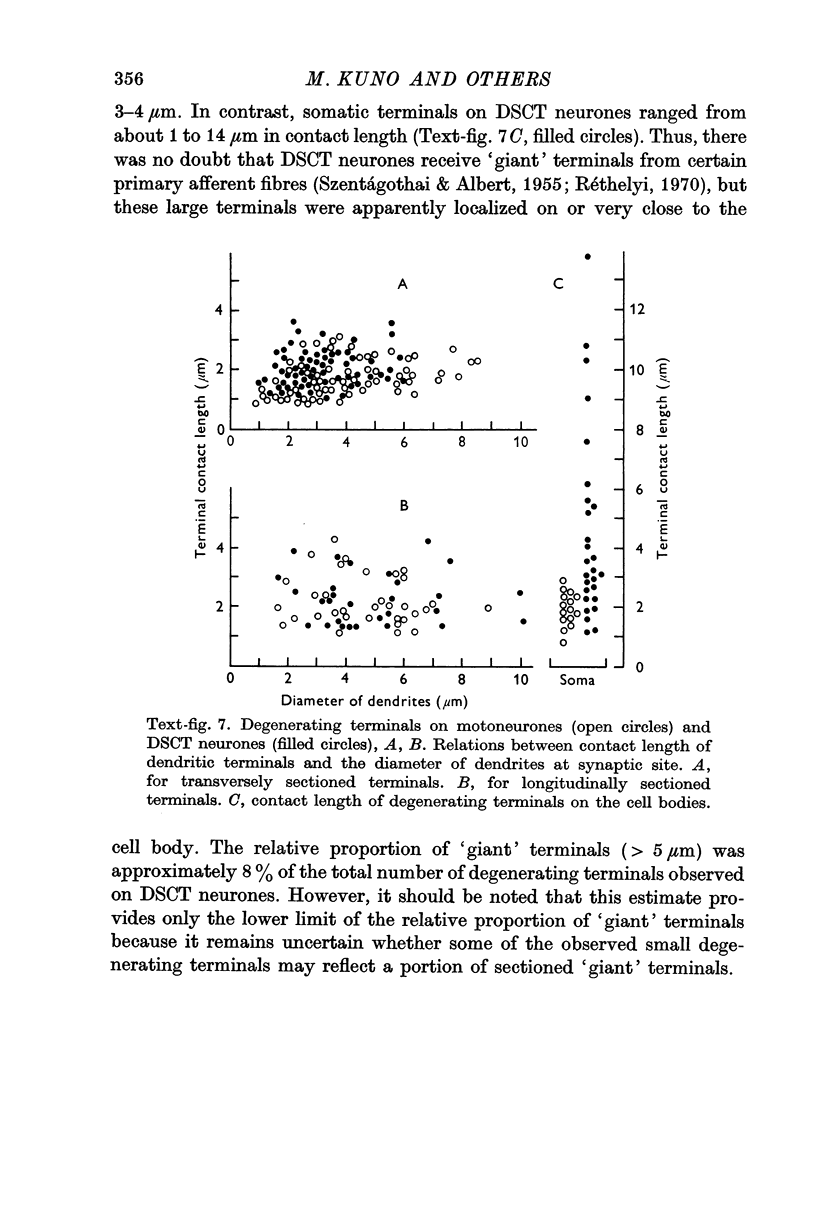
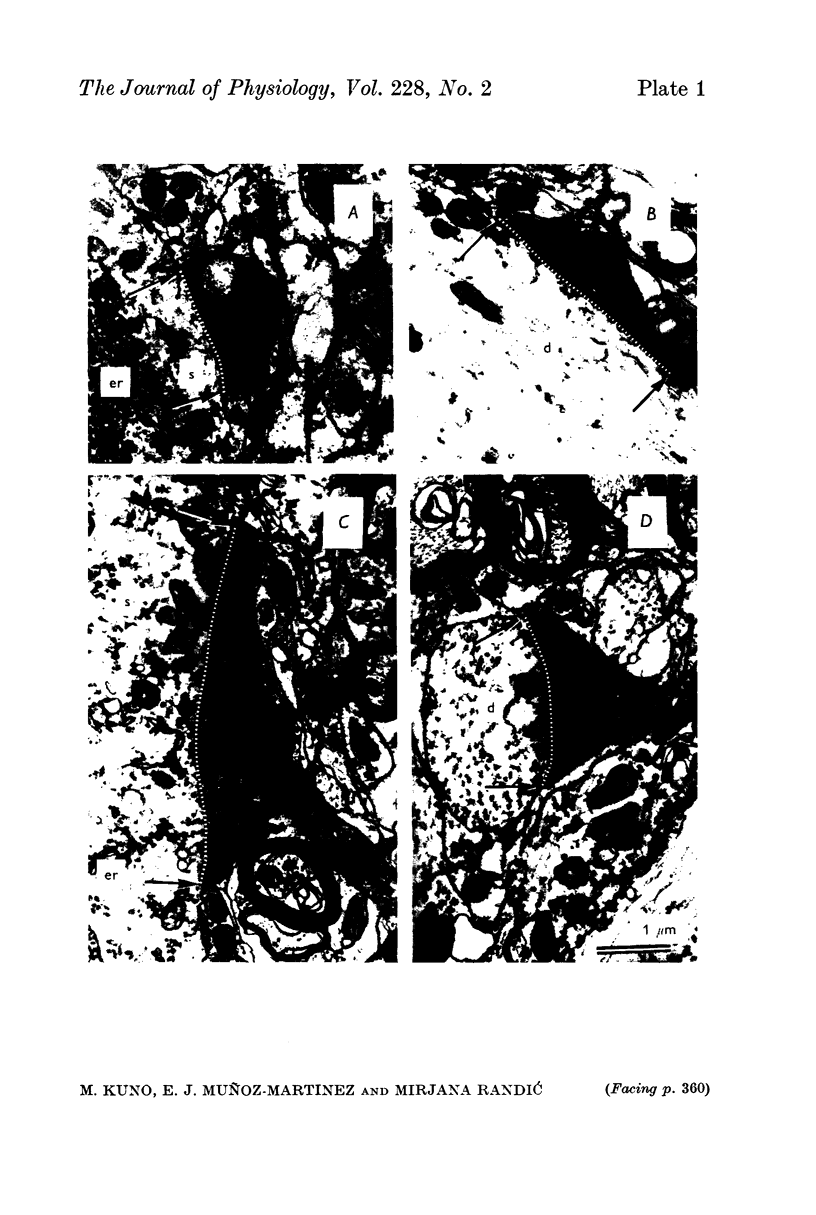
6. Degenerating terminals of primary afferent fibres on DSCT neurones and motoneurones were examined with the electron microscope after chronic section of the dorsal roots.
7. Dendritic degenerating terminals showed no significant difference in size between motoneurones and DSCT neurones. Degenerating `giant' terminals were found on DSCT neurones, but they were located only on or very close to the cell body.
8. It is concluded that the major factor responsible for a large number of `quanta' of transmitter released at synapses on DSCT neurones is the number of multiple synaptic contacts formed by one afferent fibre rather than the size of individual synapses.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship J. E., Kuno M. Analysis of spontaneous subthreshold activity in spinal motoneurons of the cat. J Neurophysiol. 1968 Mar;31(2):195–209. doi: 10.1152/jn.1968.31.2.195. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Properties of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):125–144. doi: 10.1111/j.1748-1716.1969.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Unitary components in the activation of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):145–158. doi: 10.1111/j.1748-1716.1969.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Lindstrom S., Takata M. A re-evaluation of the afterhyperpolarization mechanism in dorsal spinocerebellar tract neurons. Brain Res. 1971 Dec 24;35(2):543–546. doi: 10.1016/0006-8993(71)90497-5. [DOI] [PubMed] [Google Scholar]
- Hongo T., Okada Y. Cortically evoked pre- and postsynaptic inhibition of impulse transmission to the dorsal spinocerebellar tract. Exp Brain Res. 1967;3(2):163–177. doi: 10.1007/BF00233260. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Factors responsible for multiple discharge of neurons in Clarke's column. J Neurophysiol. 1968 Jul;31(4):624–638. doi: 10.1152/jn.1968.31.4.624. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Non-linear summation of unit synaptic potentials in spinal motoneurones of the cat. J Physiol. 1969 Apr;201(2):465–477. doi: 10.1113/jphysiol.1969.sp008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T., Weakly J. N. Post-tetanic hyperpolarization produced by an electrogenic pump in dorsal spinocerebellar tract neurones of the cat. J Physiol. 1970 Nov;210(4):839–855. doi: 10.1113/jphysiol.1970.sp009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Muñoz-Martinez E. J., Randić M. Sensory inputs to neurones in Clarke's column from muscle, cutaneous and joint receptors. J Physiol. 1973 Jan;228(2):327–342. doi: 10.1113/jphysiol.1973.sp010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A. ASCENDING SPINAL HINDLIMB PATHWAYS IN THE CAT. Prog Brain Res. 1964;12:135–163. doi: 10.1016/s0079-6123(08)60621-4. [DOI] [PubMed] [Google Scholar]
- MCEWEN L. M. The effect on the isolated rabbit heart of vagal stimulation and its modification by cocaine, hexamethonium and ouabain. J Physiol. 1956 Mar 28;131(3):678–689. doi: 10.1113/jphysiol.1956.sp005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J. Fine structure of synapses in the dorsal nucleus of the lateral geniculate body of normal and blinded rats. Z Zellforsch Mikrosk Anat. 1967;76(1):116–146. doi: 10.1007/BF00337036. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- OSCARSSON O. FUNCTIONAL ORGANIZATION OF THE SPINO- AND CUNEOCEREBELLAR TRACTS. Physiol Rev. 1965 Jul;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Réthelyi M. Ultrastructural synaptology of Clarke's column. Exp Brain Res. 1970;11(2):159–174. doi: 10.1007/BF00234320. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J., ALBERT A. The synaptology of Clarke's column. Acta Morphol Acad Sci Hung. 1955;5(1-2):43–51. [PubMed] [Google Scholar]
- Smith C. A., Rasmussen G. L. Degeneration in the efferent nerve endings in the cochlea after axonal section. J Cell Biol. 1965 Jul;26(1):63–77. doi: 10.1083/jcb.26.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN CREVEL, VERHAART W. J. THE RATE OF SECONDARY DEGENERATION IN THE CENTRAL NERVOUS SYSTEM. I. THE PYRAMIDAL TRACT OF THE CAT. J Anat. 1963 Jul;97:429–449. [PMC free article] [PubMed] [Google Scholar]