Abstract
1. The relation between m.e.p.p. frequency (F) and [Ca] was studied at the mouse neuromuscular junction, at varied concentrations of K+ and at nerve terminals depolarized by focal depolarization.
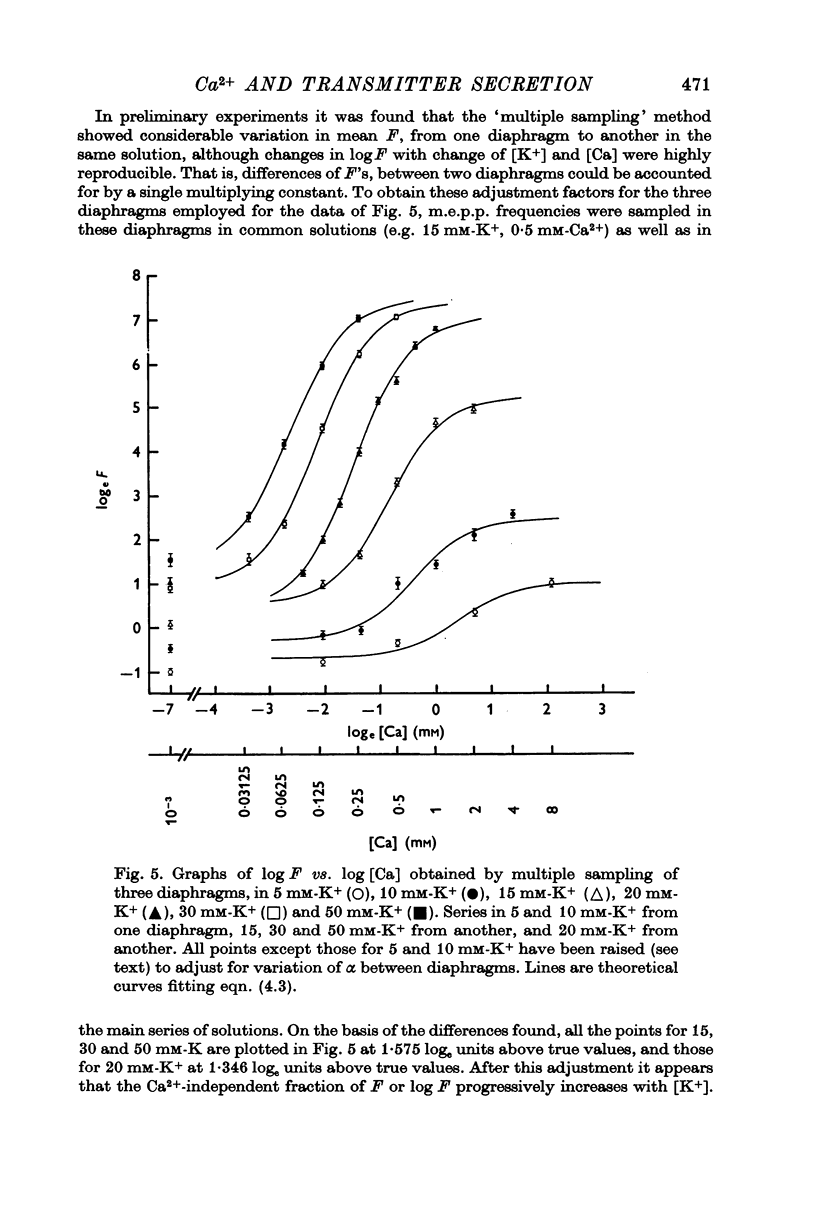
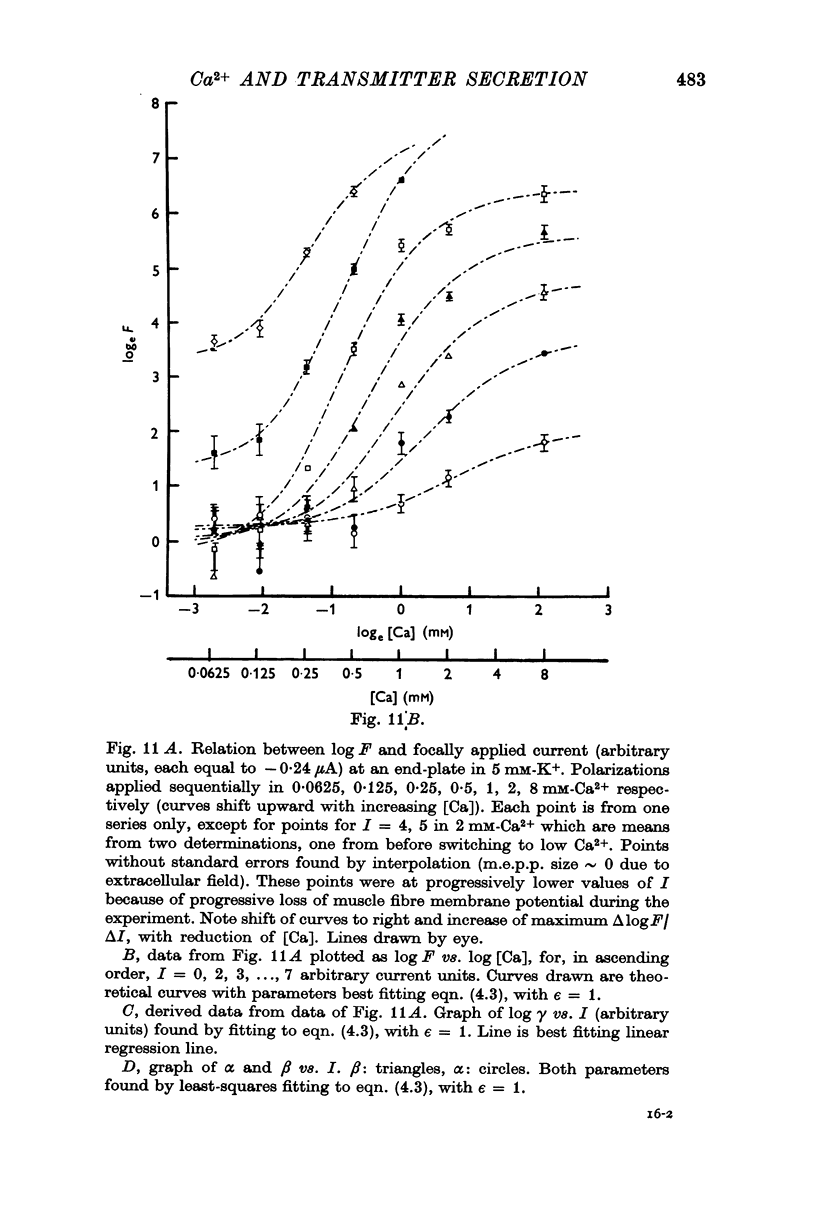
2. Under all conditions the relation between log F and log [Ca] was sigmoid, with a maximum slope that increased with depolarization or raised [K+]. In addition, depolarization or raised K+ caused a progressive shift of the sigmoid curve upward and to the left (to reduced log [Ca]) and increased the range over which log F could be altered by [Ca].
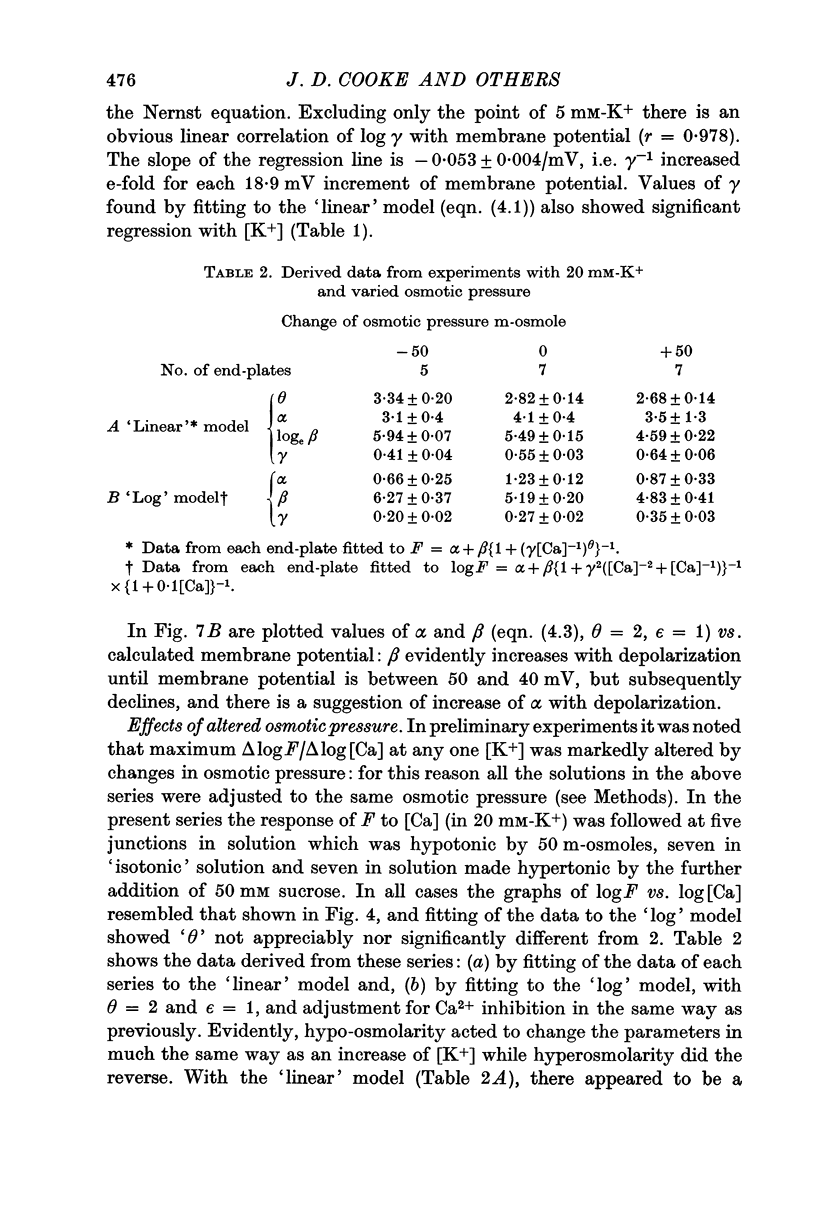
3. Reduction of osmotic pressure changed the relation between log F and log [Ca] in the same way as increase of depolarization, while increase of osmotic pressure did the opposite.
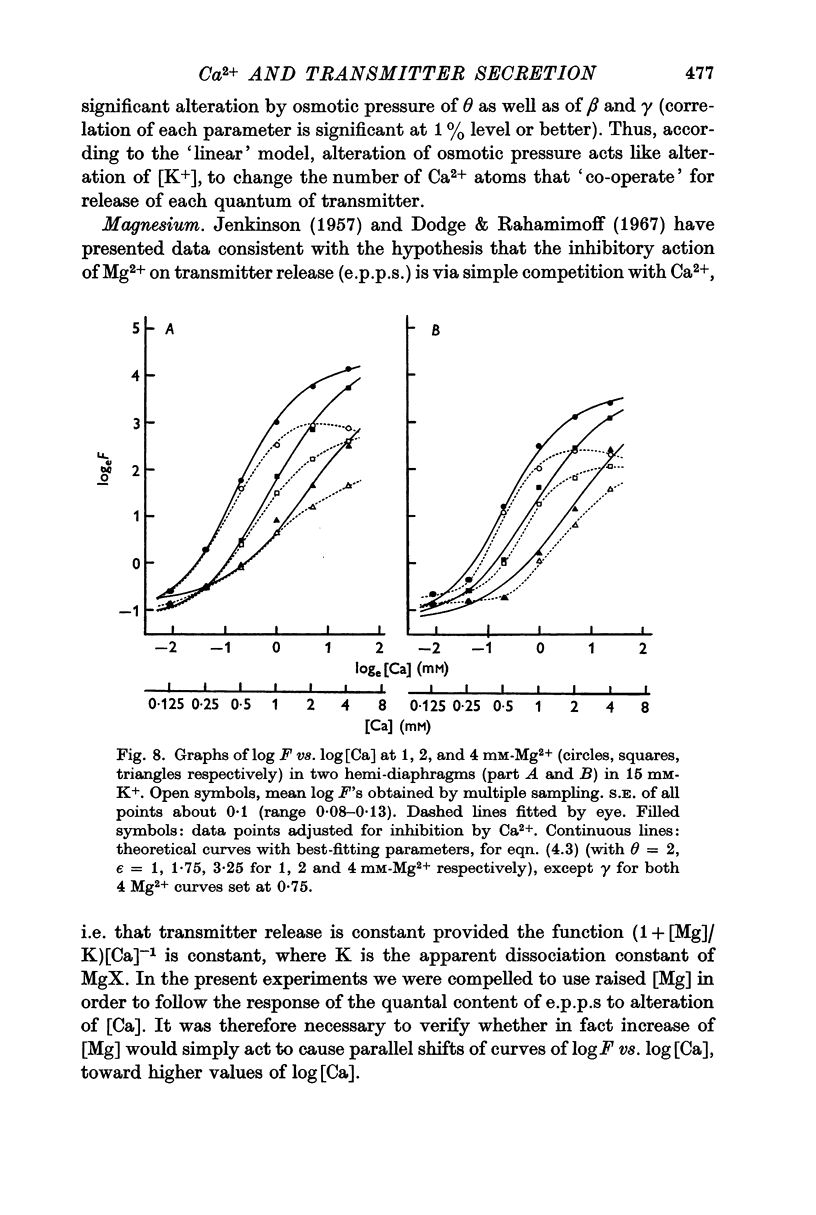
4. Raised [Mg] acted in two ways: (a) to shift the curve of log F vs. log [Ca] to the right and (b) to reduce maximum Δ log F/Δ log [Ca] without altering the range of log F sensitive to [Ca].
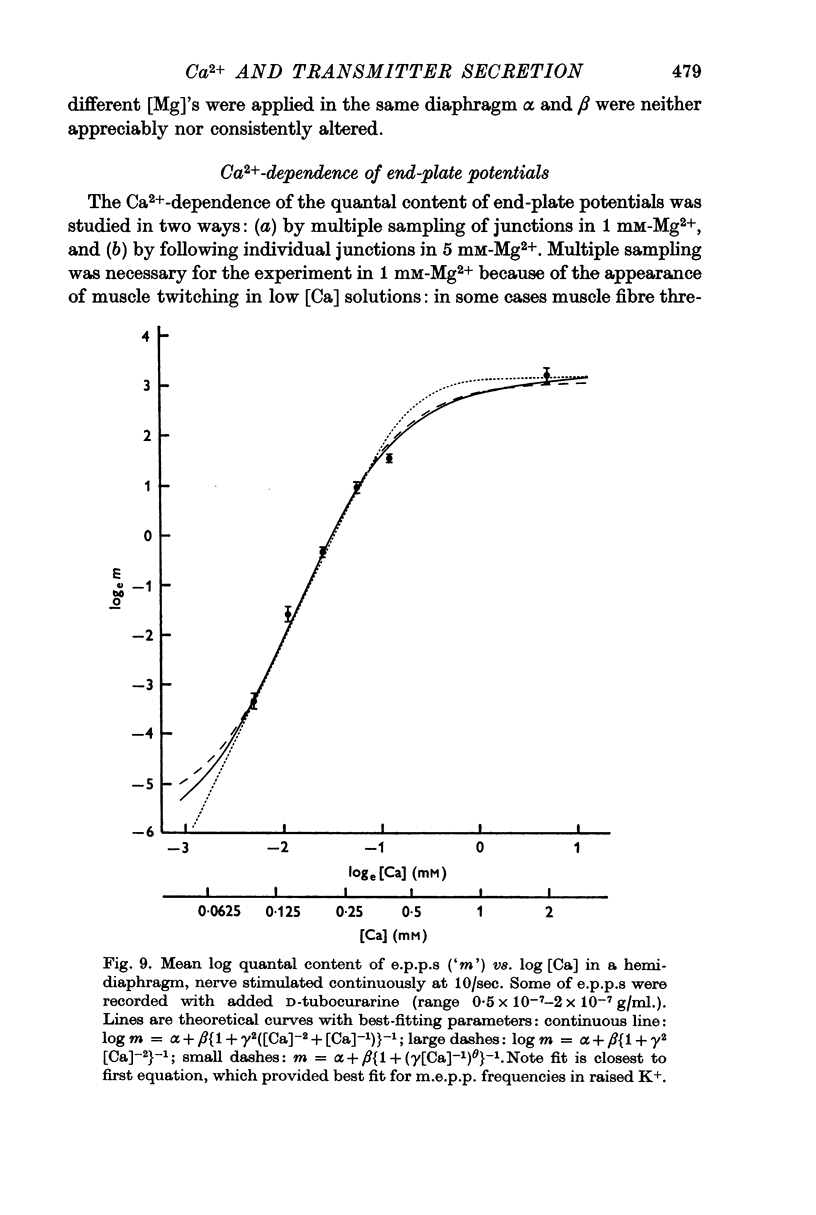
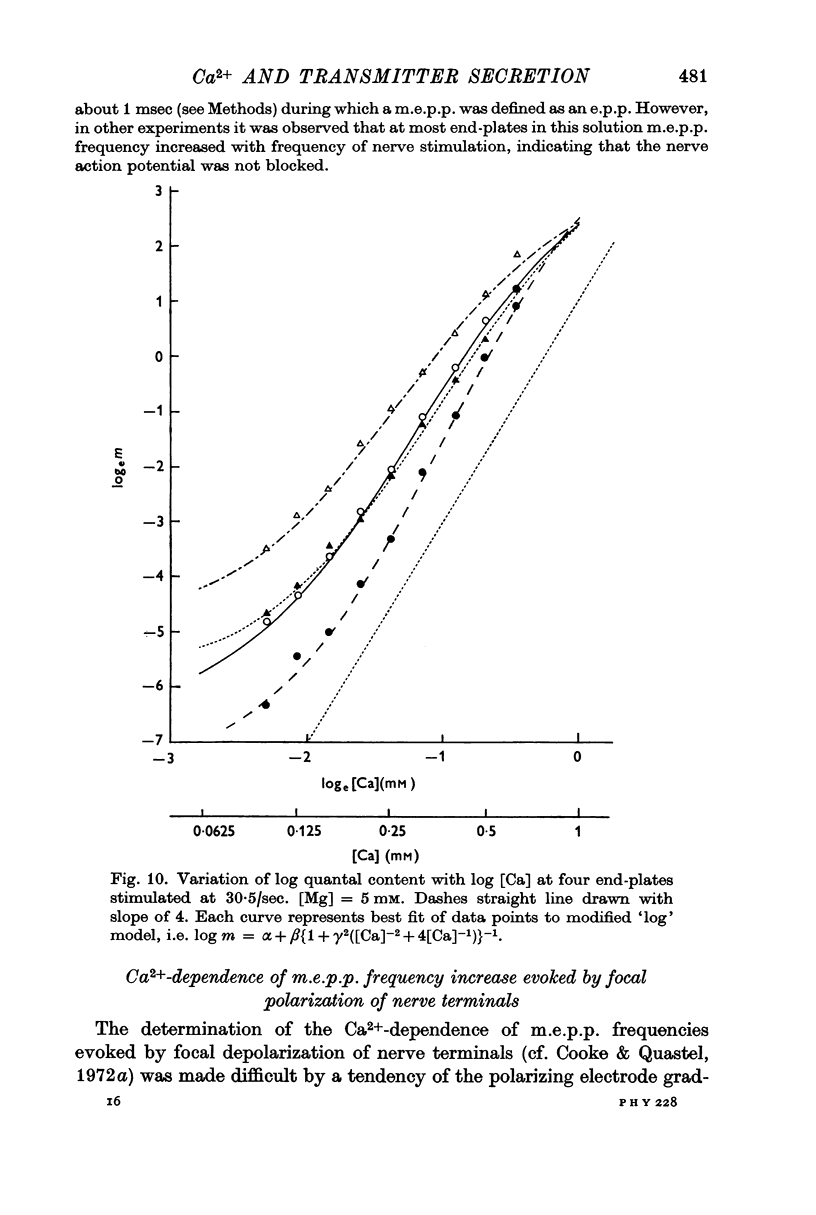
5. The relation between log quantal content of e.p.p.s and log [Ca] was similar to that between log m.e.p.p. frequency and log [Ca].
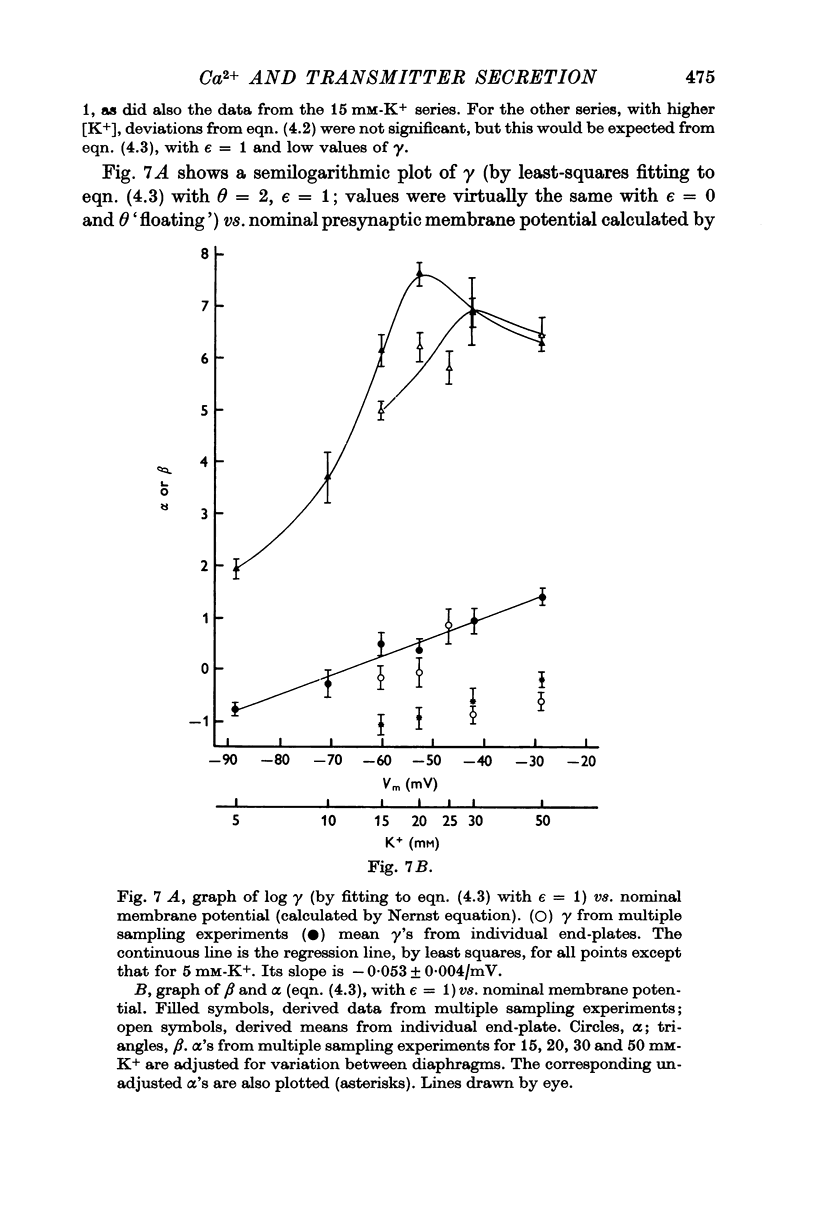
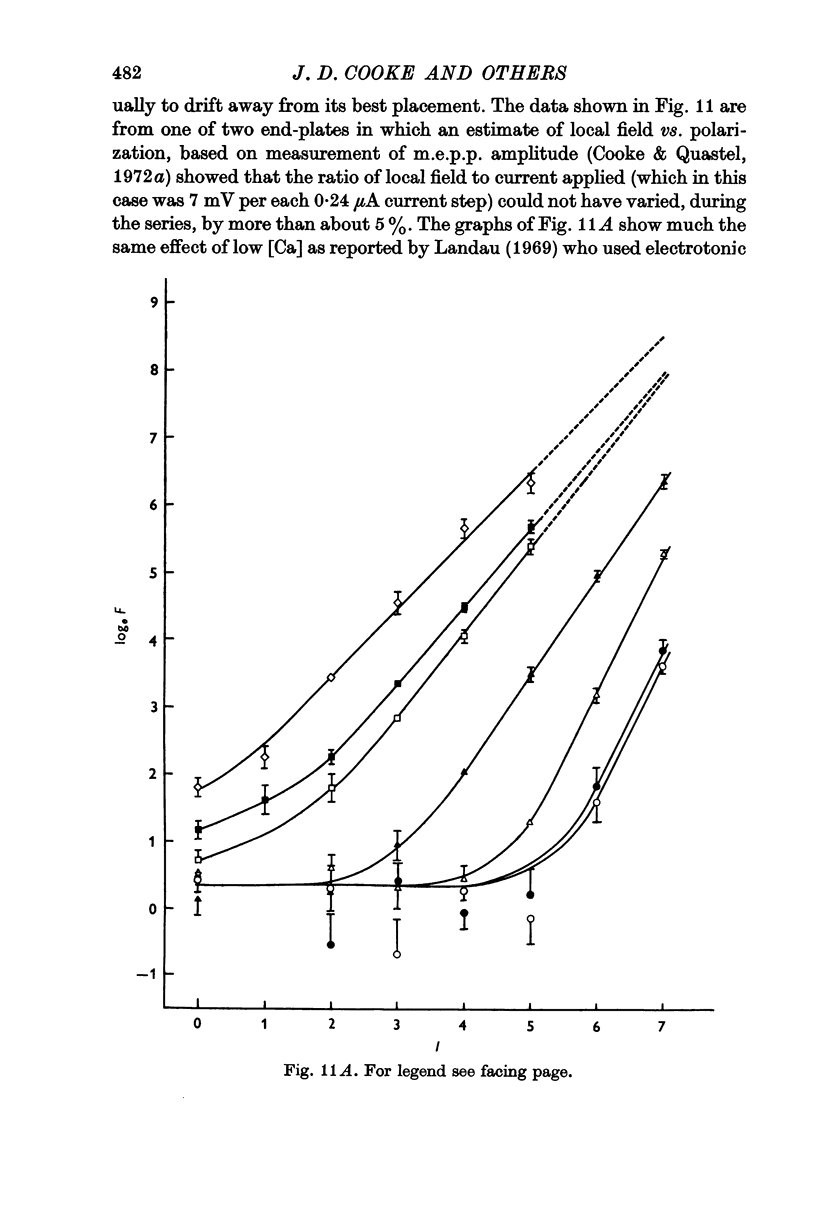
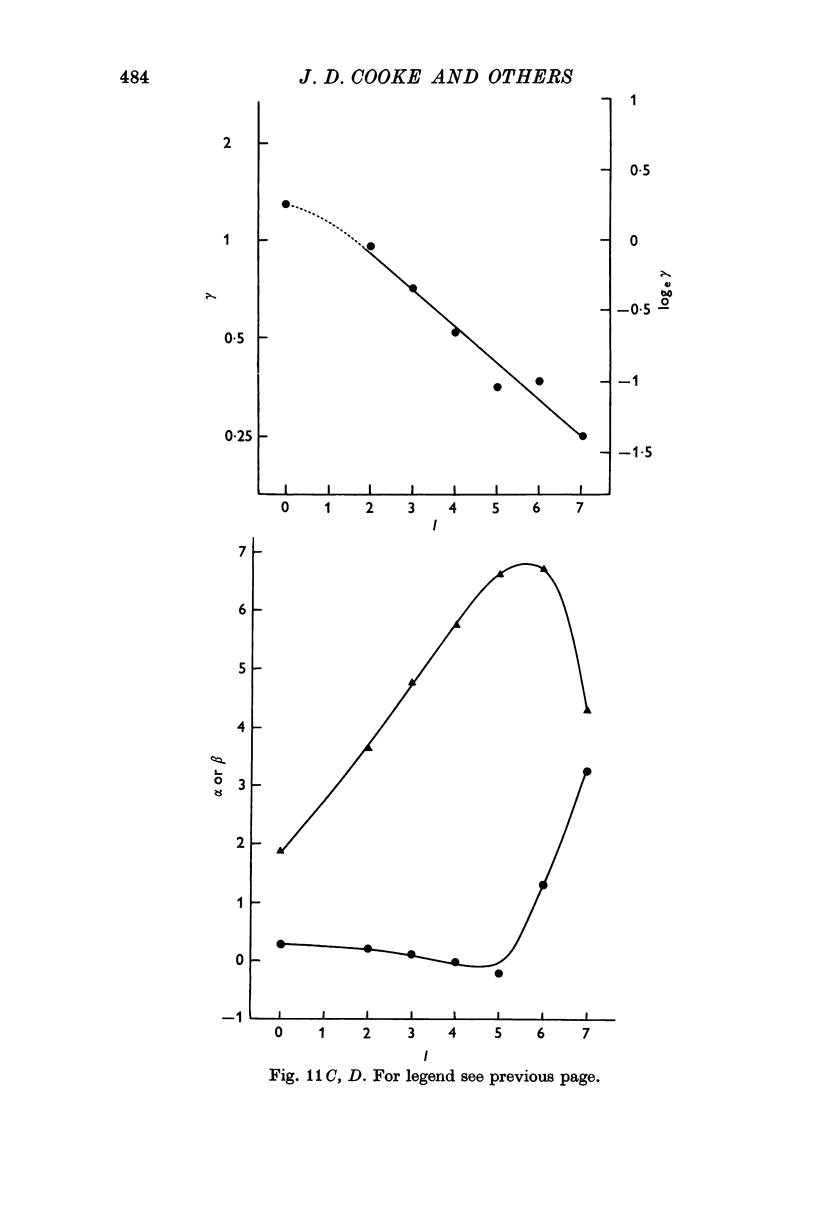
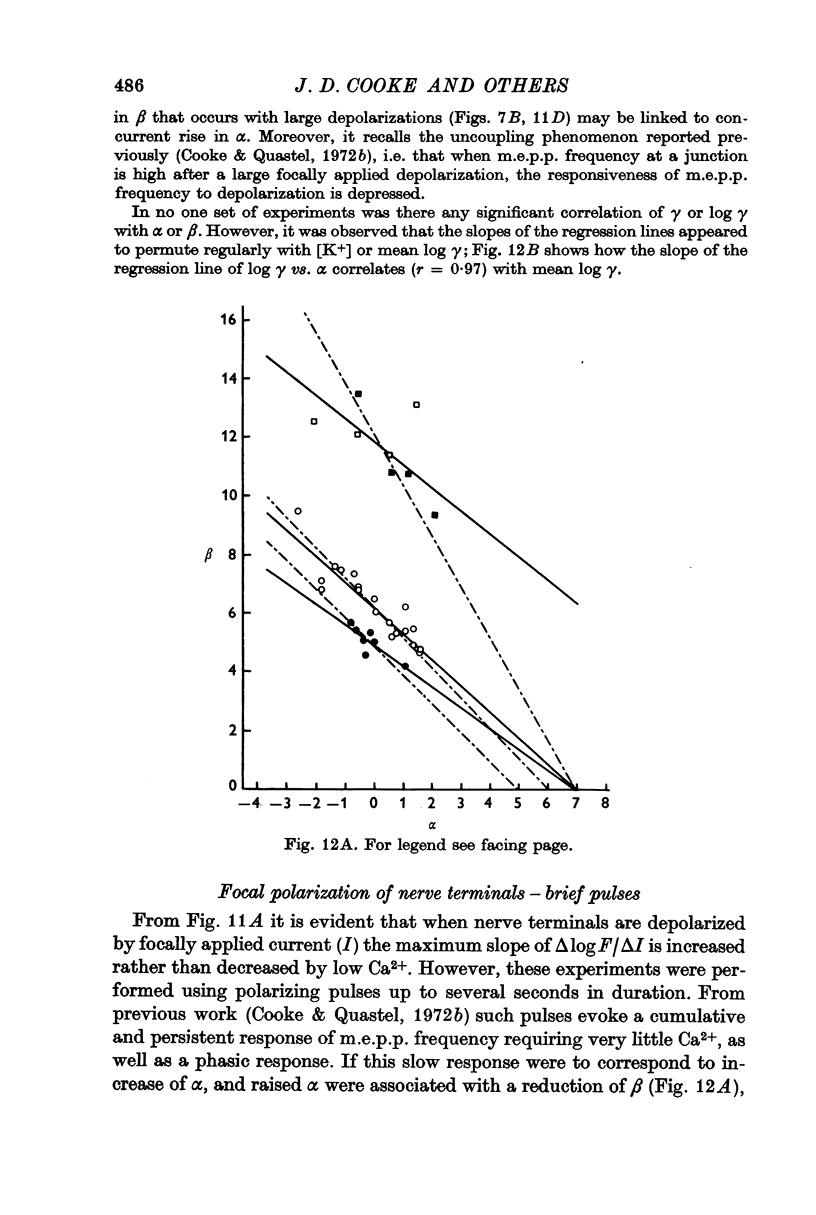
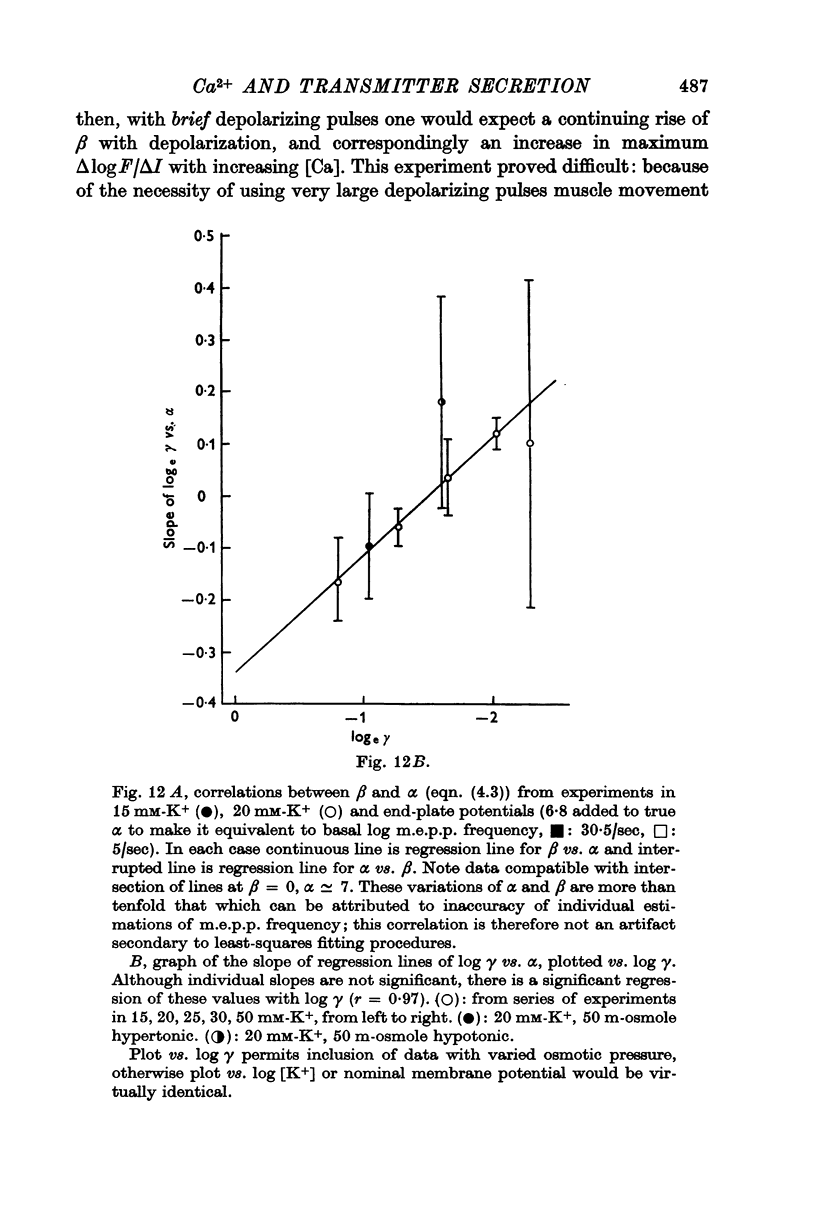
6. Individual nerve terminals varied in both Ca-dependent and Ca-independent fractions of log F; a large Ca-independent portion appears to be associated with a low Ca-dependent portion and vice versa. With large prolonged depolarization the Ca-independent portion was increased, apparently at the expense of the Ca-dependent portion.
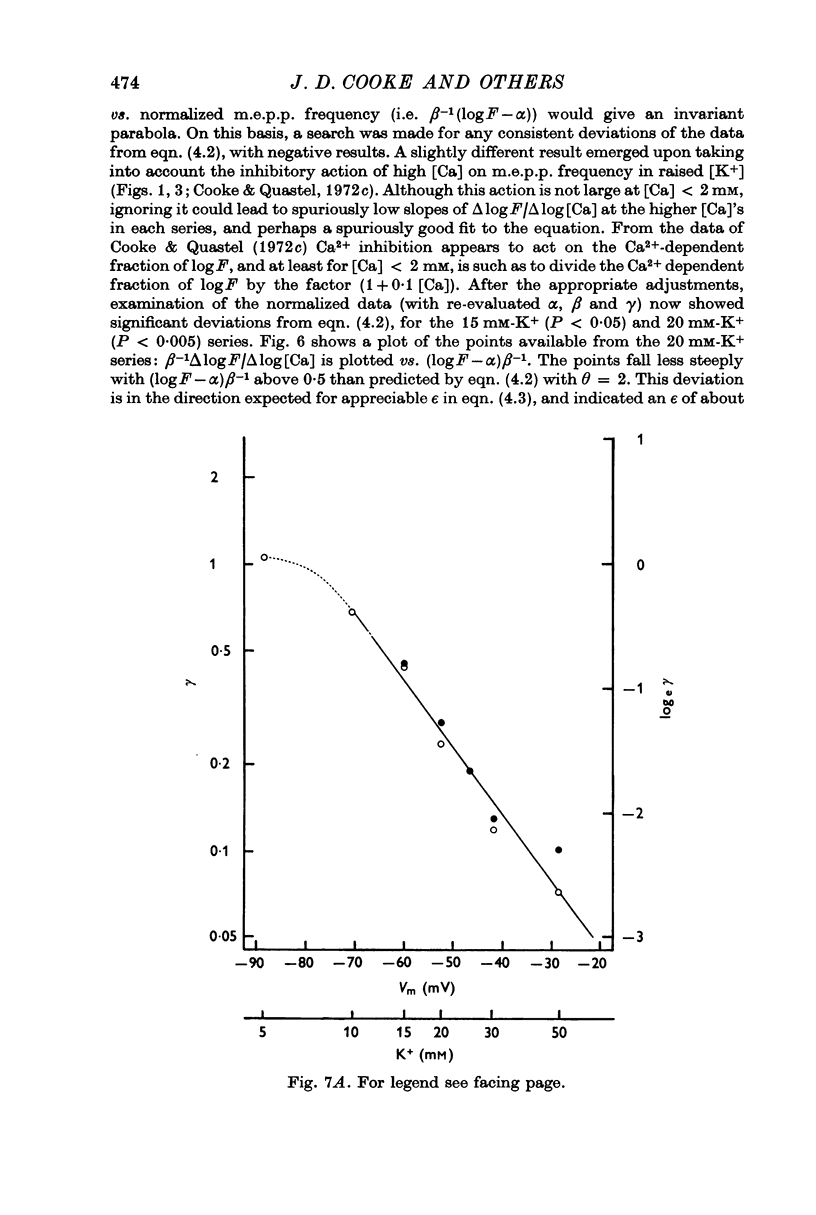
7. The results of all experiments were summarized in terms of parameters found by fitting the observed log release —log [Ca] curves to two theoretical equations, each derived on the basis of a model: (a) all-or-nothing activation of release probability by Ca-complex(es) and (b) graded activation of release probability by Ca complex(es).
8. On the basis of the all-or-nothing model, from which follows alinear relation between F and amounts of Ca complex(es), the number of Ca2+ atoms that `cooperate' to mediate release appeared to increase progressively with presynaptic depolarization, to a value of 4 or more with a presynaptic action potential.
9. On the basis of the graded activation model, which predicts an exponential relation between F and amount of Ca complex, the number of Ca2+ atoms that combine with Ca receptor appears to be independent of presynaptic depolarization.
10. Various models which could account for the data are discussed. It was concluded that all the data are consistent with a model in which:
(i) quantal release probability is continuously graded with the amount of a simple Ca complex (CaX) inside the nerve terminal.
(ii) Ca entry is governed by presynaptic membrane potential (increasing exponentially with depolarization) and by the amount of a Ca complex (Ca2Y) on or in the membrane.
(iii) Mg2+ competes with Ca2+ at both receptors, X and Y.
(iv) The internal Ca receptor X is also increased by presynaptic depolarization.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke J. D., Quastel D. M. Cumulative and persistent effects of nerve terminal depolarization on transmitter release. J Physiol. 1973 Jan;228(2):407–434. doi: 10.1113/jphysiol.1973.sp010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. The specific effect of potassium on transmitter release by motor nerve terminals and its inhibition by calcium. J Physiol. 1973 Jan;228(2):435–458. doi: 10.1113/jphysiol.1973.sp010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. The effect of calcium ions on the motor end-plate potentials. J Physiol. 1952 Apr;116(4):507–515. doi: 10.1113/jphysiol.1952.sp004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J. N. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969 May;201(3):515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M. The interaction of presynaptic polarization with calcium and magnesium in modifying spontaneous transmitter release from mammalian motor nerve terminals. J Physiol. 1969 Aug;203(2):281–299. doi: 10.1113/jphysiol.1969.sp008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Okamoto K. Presynaptic action of central depressant drugs: inhibition of depolarization-secretion coupling. Can J Physiol Pharmacol. 1972 Mar;50(3):279–284. doi: 10.1139/y72-042. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]


