Abstract
1. As a preliminary to chemical studies an estimate has been made of the equilibrium dissociation constant (K) for the interaction of tetrodotoxin (TTX) with the non-myelinated fibres of the rabbit desheathed vagus nerve.
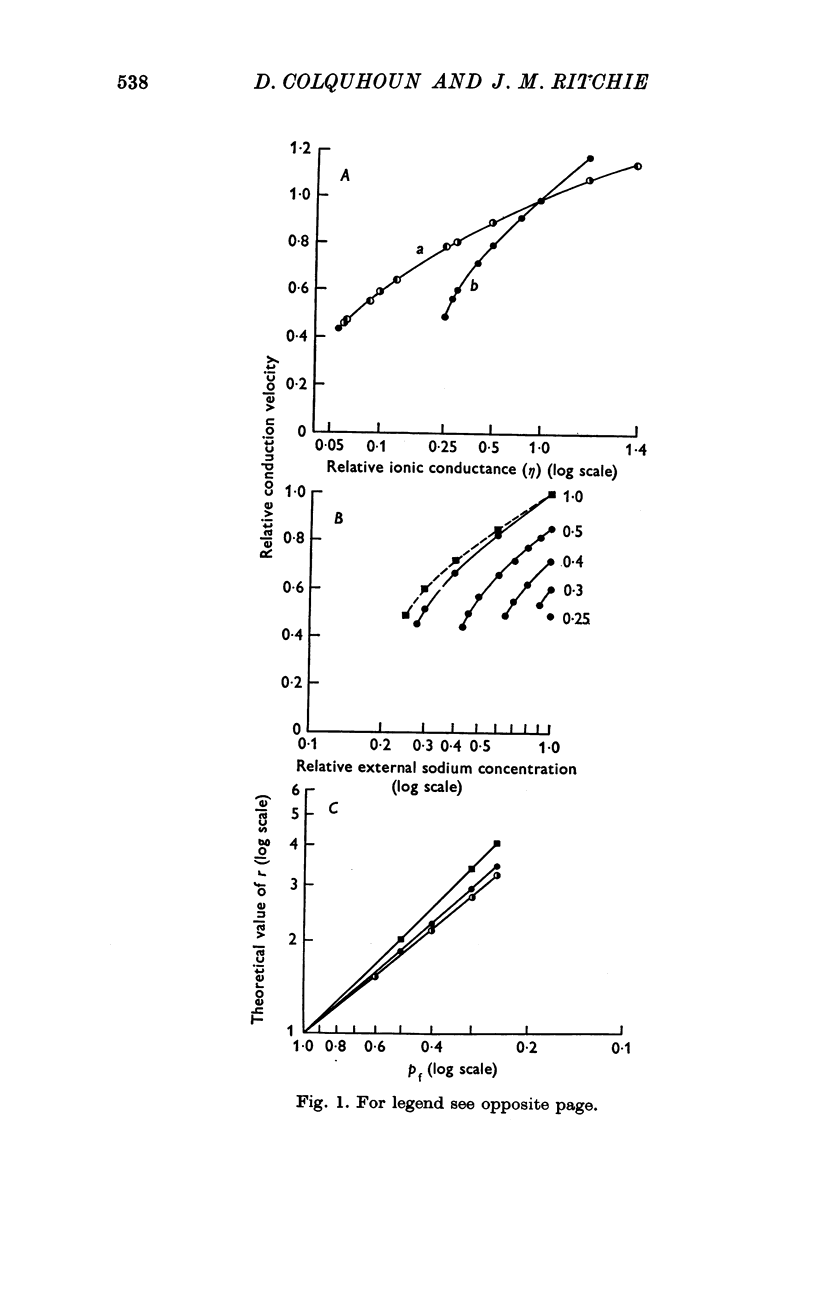
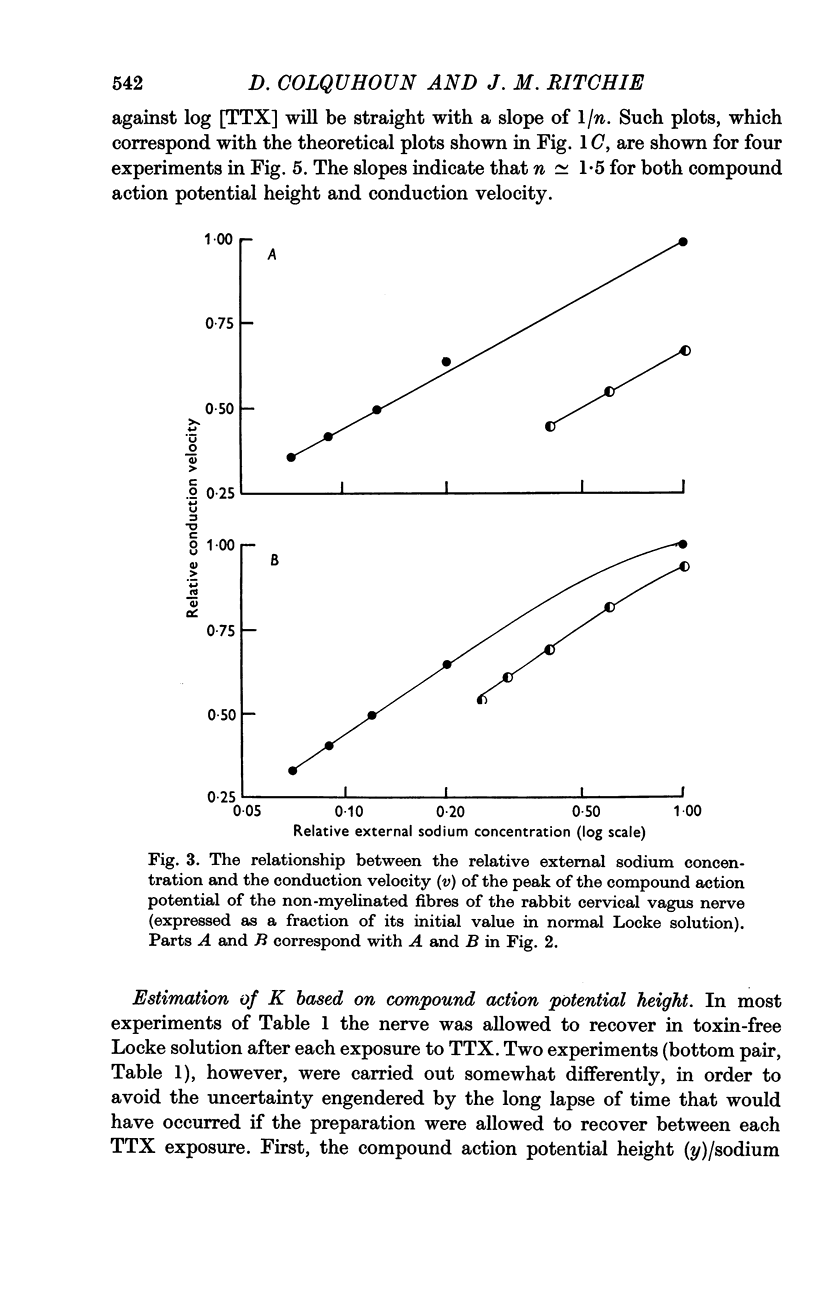
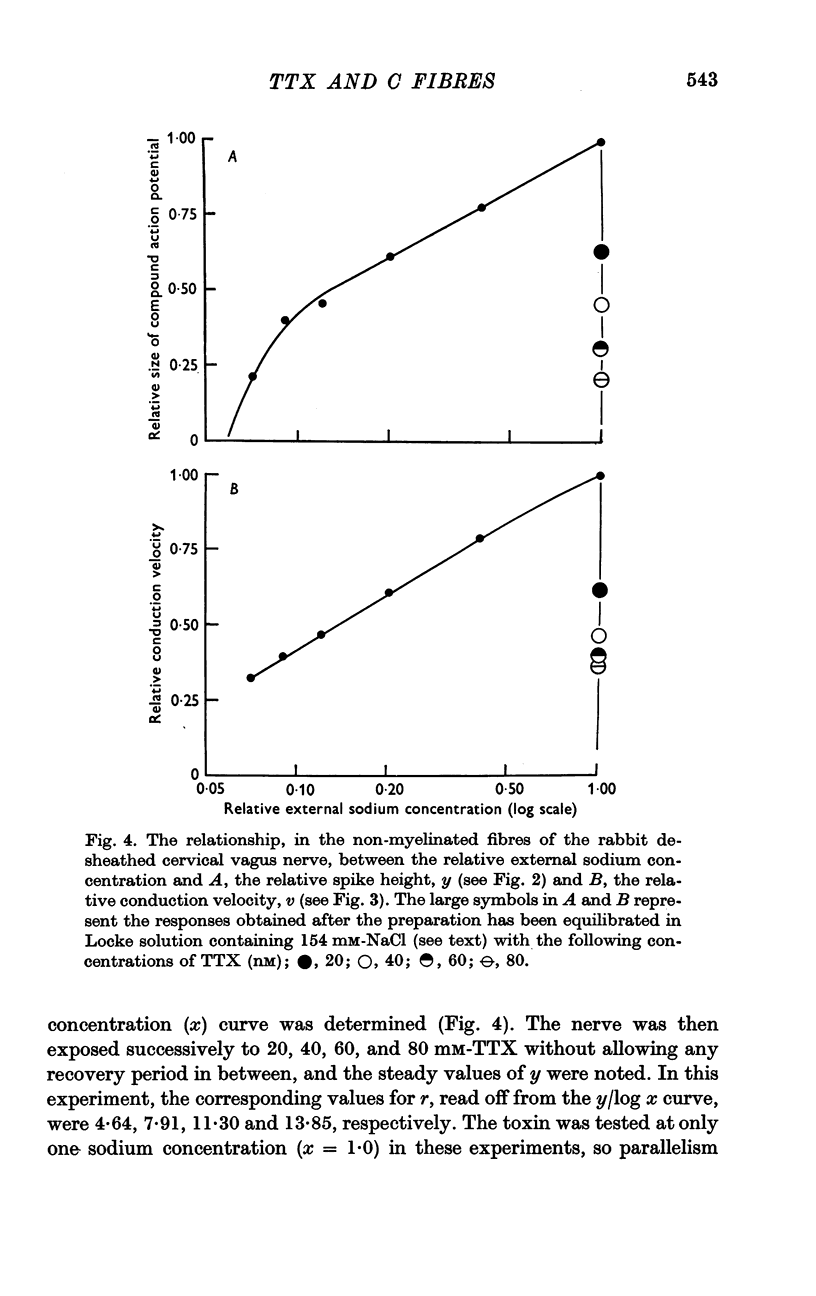
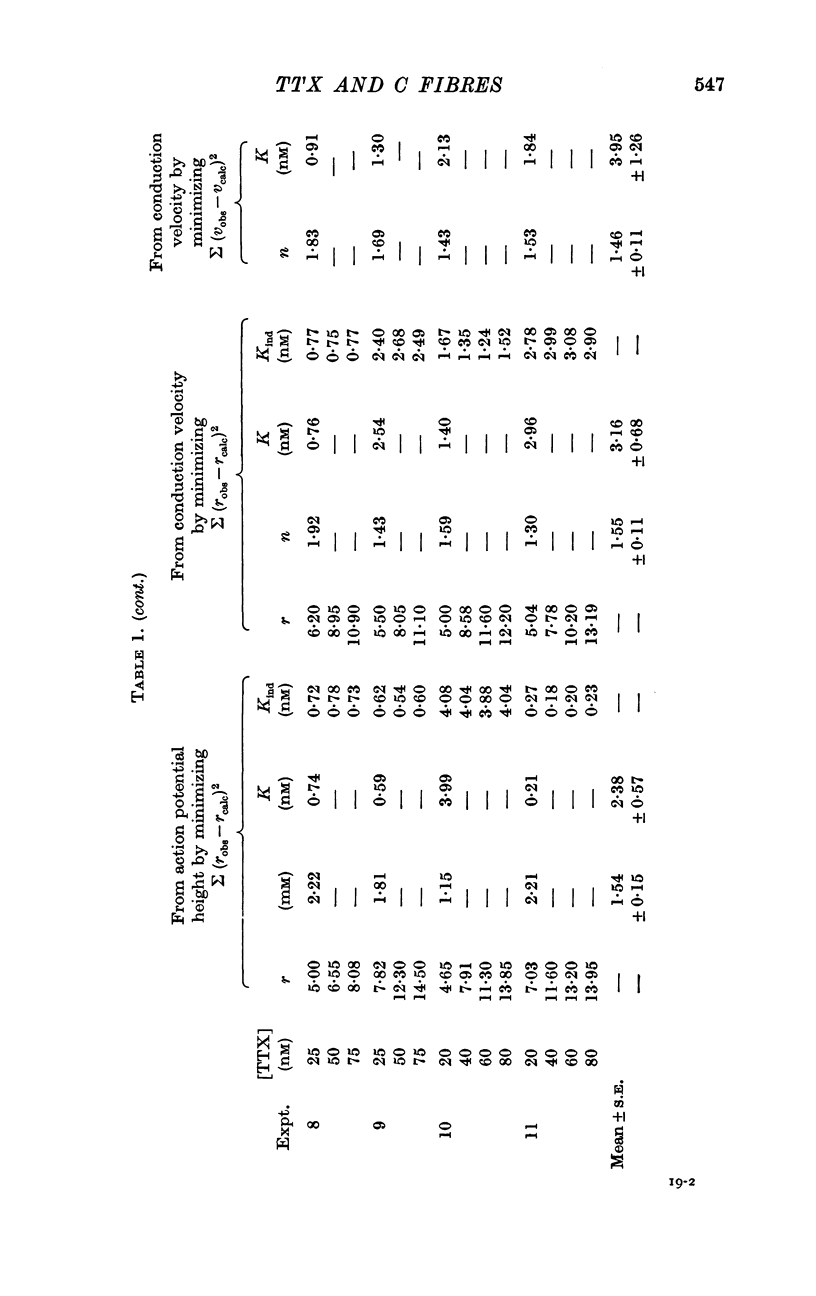
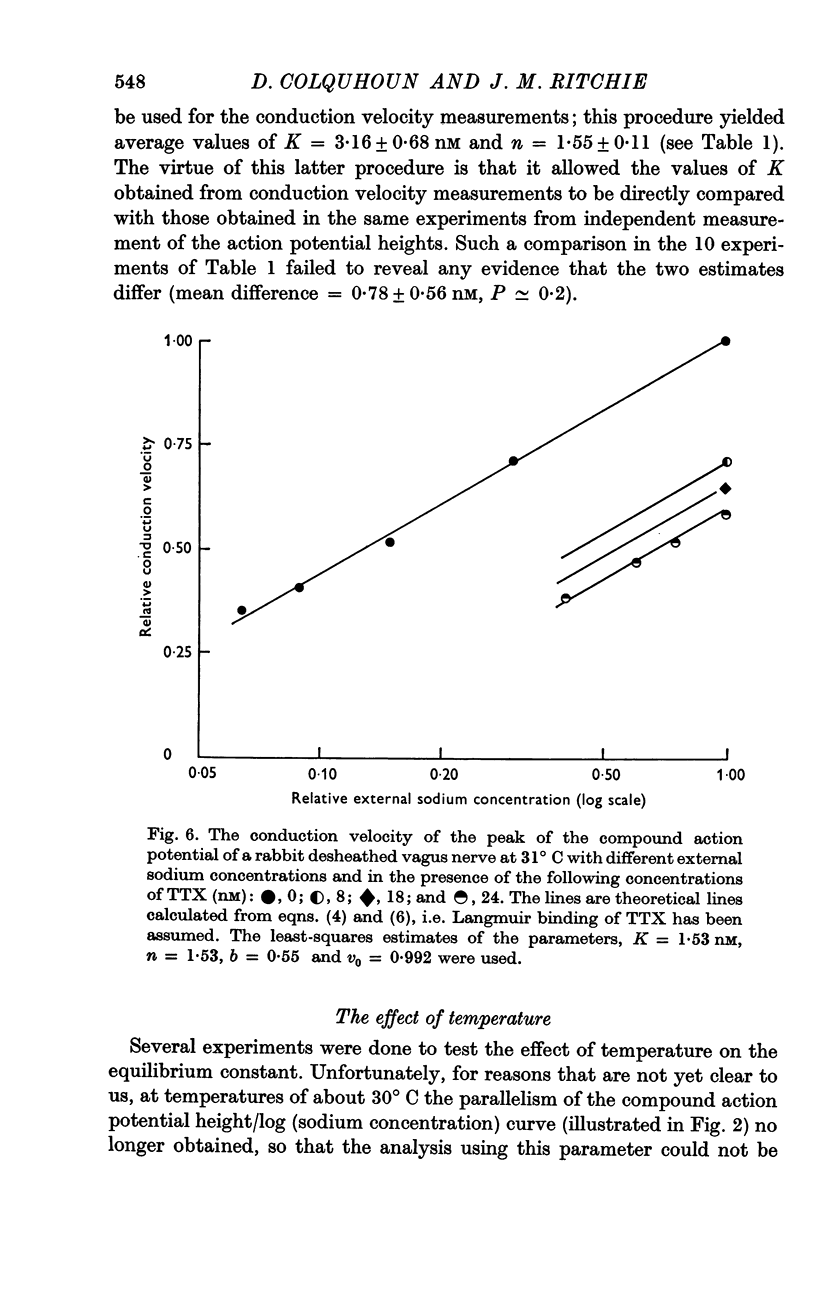
2. TTX causes a parallel shift to the right of the curves obtained when either the height or the conduction velocity of the compound action potential are plotted against the logarithm of the external sodium concentration.
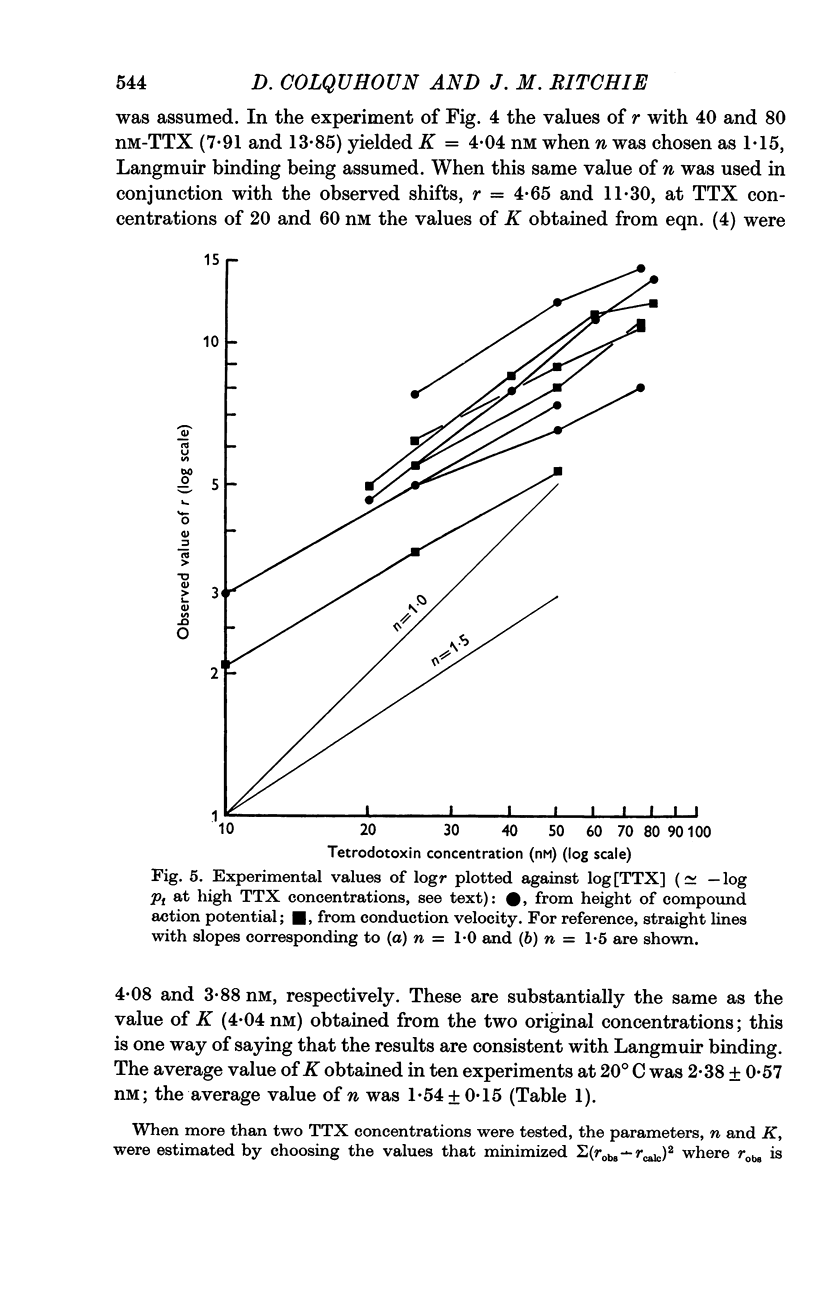
3. A model has been formulated based on the independence principle and the Hodgkin—Huxley theory, and the experimental results shown to be consistent with it. On this basis, and on the assumptions that one TTX molecule blocks one sodium channel, and that binding is Langmuir, K was estimated to be about 3-5 nM at about 20° C. Other simple non-Langmuir models gave essentially similar low values for K.
4. An alternative method of computing K that makes rather different assumptions gives a similar low value.
5. Despite the low value for K, a TTX concentration of at least 100 nM is needed to block conduction completely and this seems to be related to the fact that conduction is not completely blocked until the external sodium concentration falls below 7% of its value in normal Locke.
6. The minimum sodium concentration needed to support conduction increased with temperature.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M., Rojas E. The binding of tetrodotoxin to nerve membranes. J Physiol. 1971 Feb;213(1):235–254. doi: 10.1113/jphysiol.1971.sp009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]


