Abstract
1. The respiratory response, measured directly as tidal volume or indirectly by using integrated peak phrenic activity, to intermittent electrical stimulation of the carotid sinus nerve was determined in anaesthetized cats.
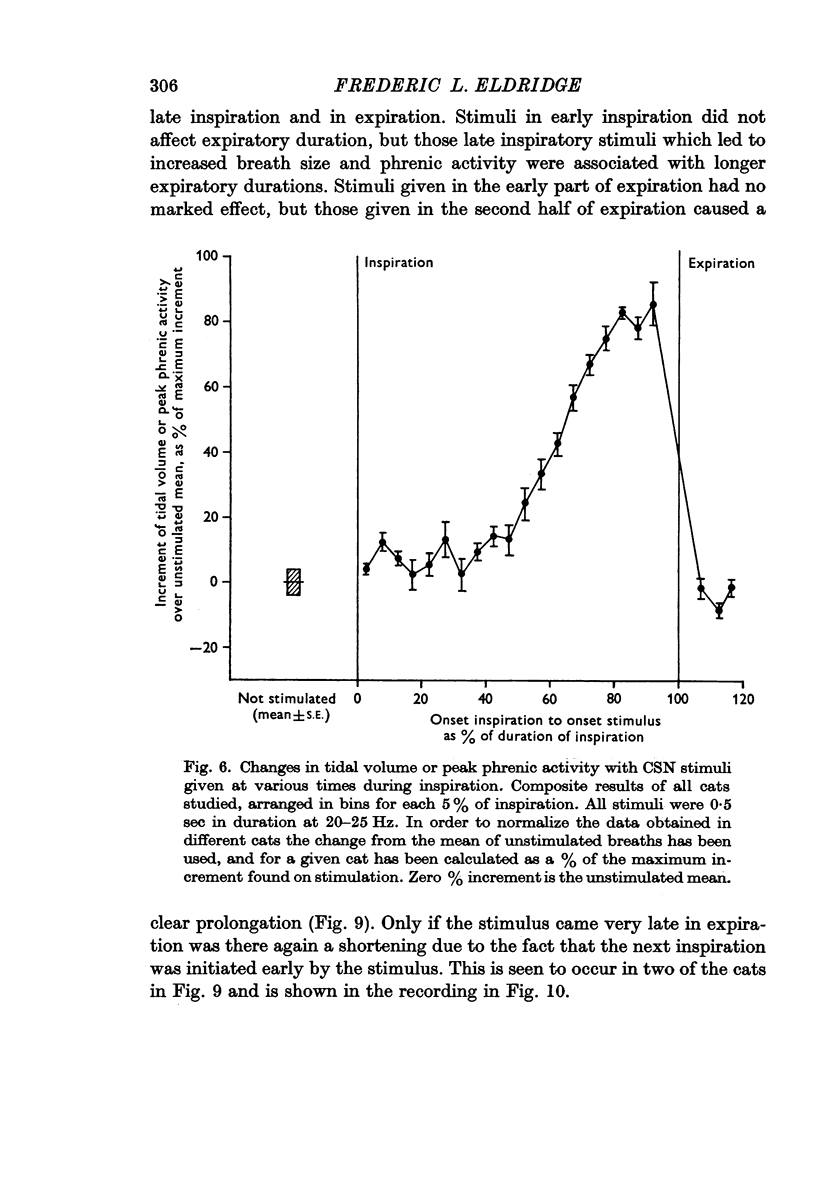
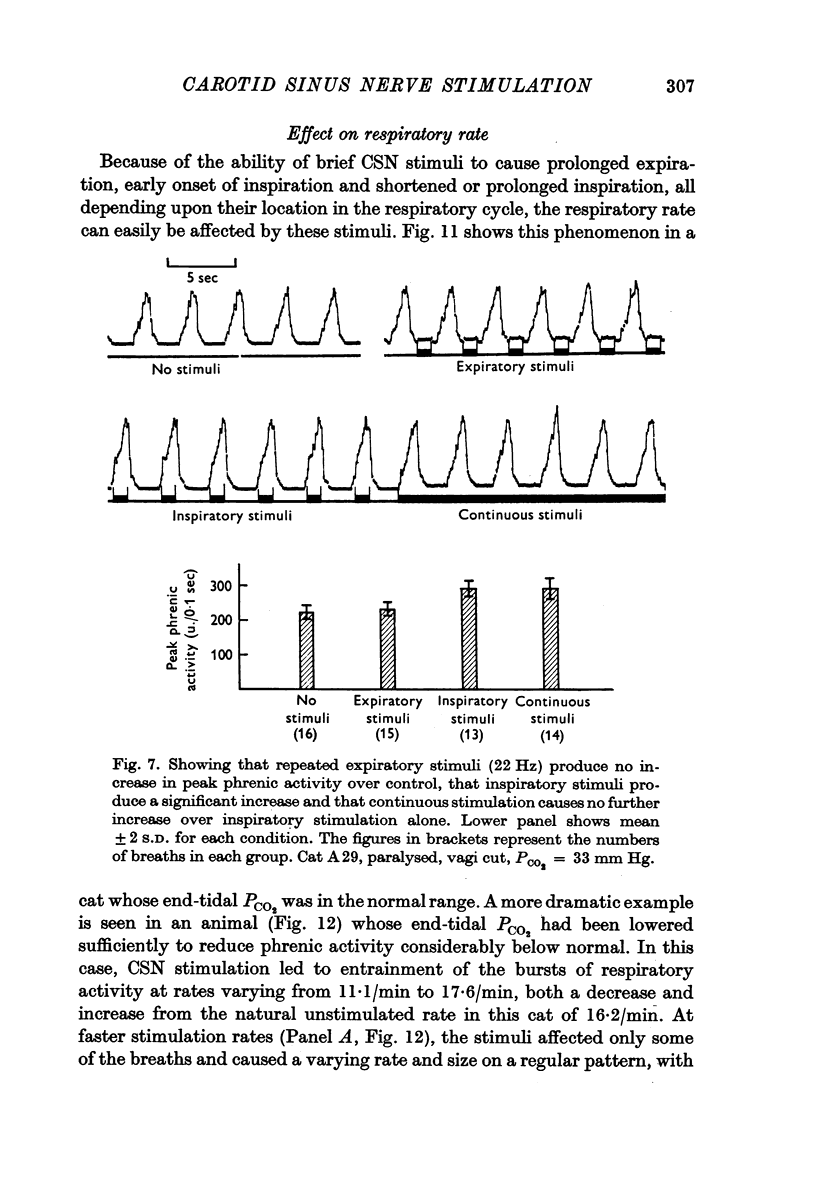
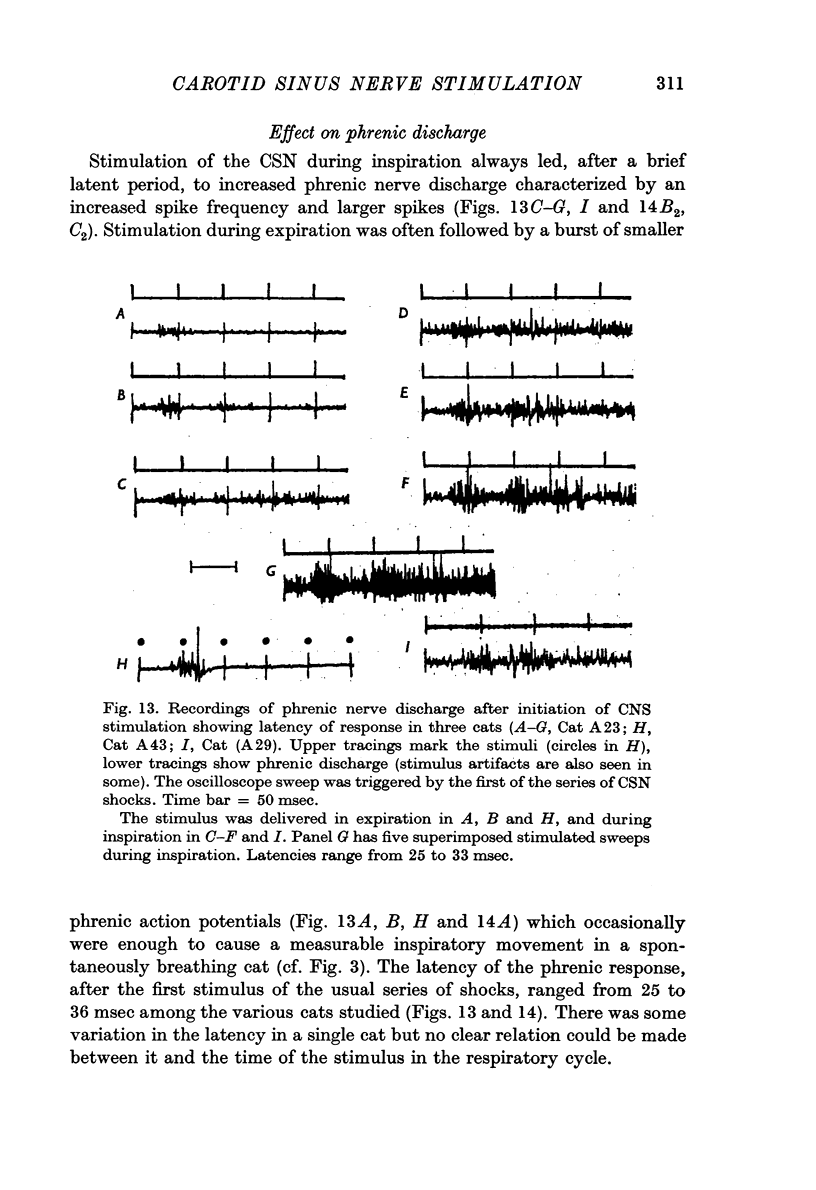
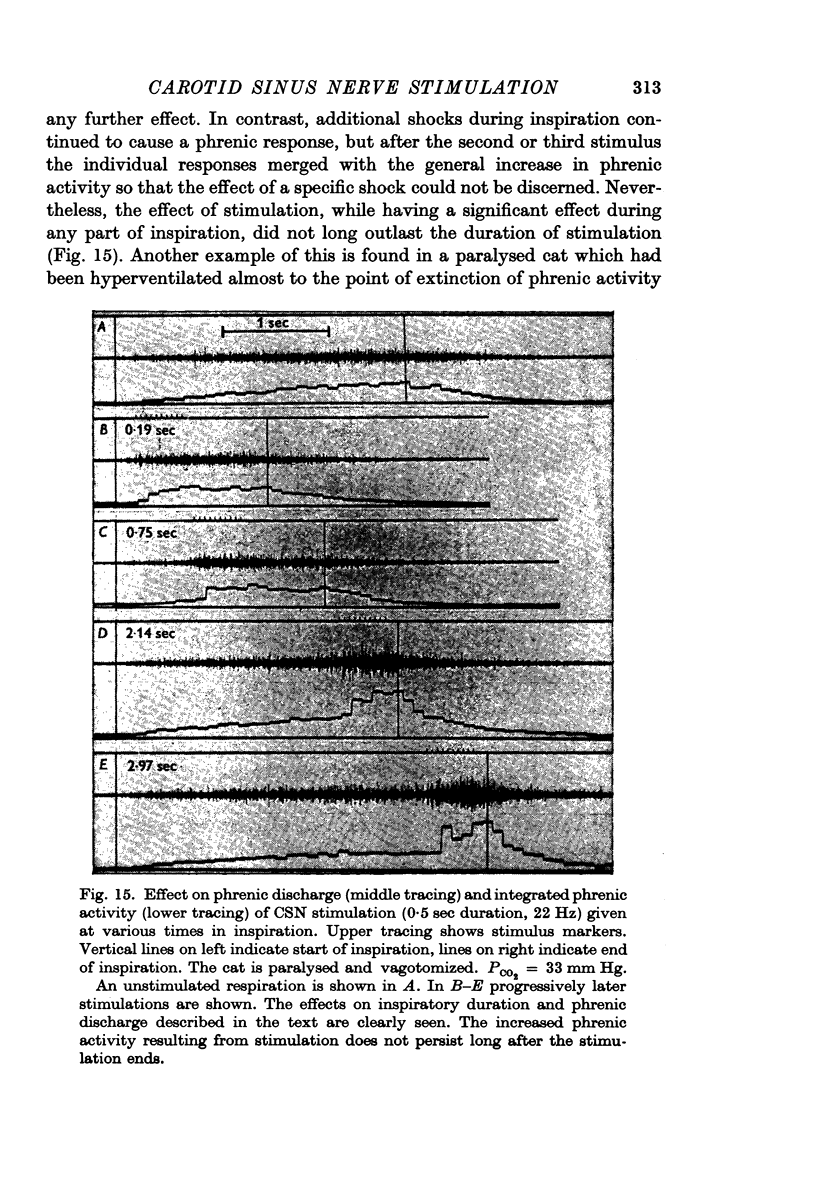
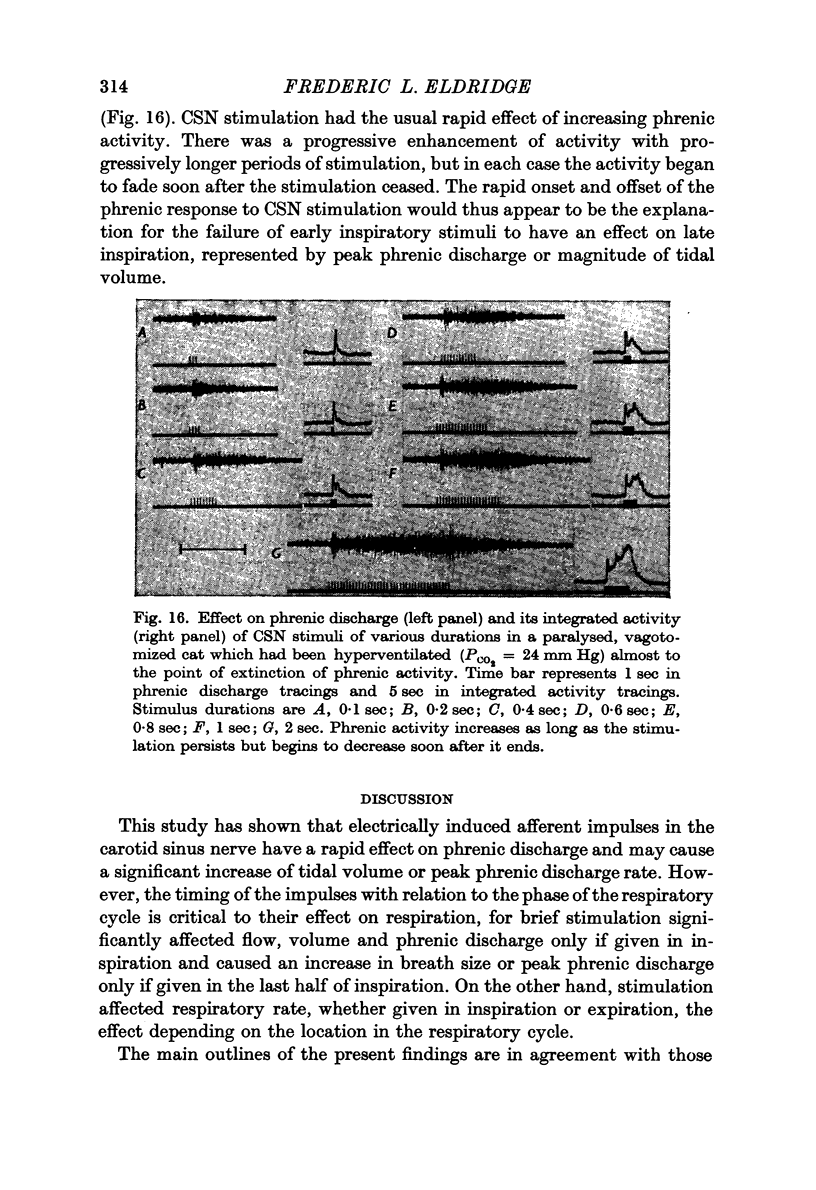
2. Stimulation at rates of 20-25 Hz for 0·5 sec had a rapid effect, increasing inspiratory airflow and phrenic discharge, but only if applied during inspiration. An increase in tidal volume or peak level of integrated phrenic discharge occurred only if the stimulus was exhibited during the second half of inspiration. Continuous stimulation had no greater effect on size or frequency of breathing than did intermittent inspiratory stimuli alone. Stimulation during expiration had no effect on the form or magnitude of subsequent breaths.
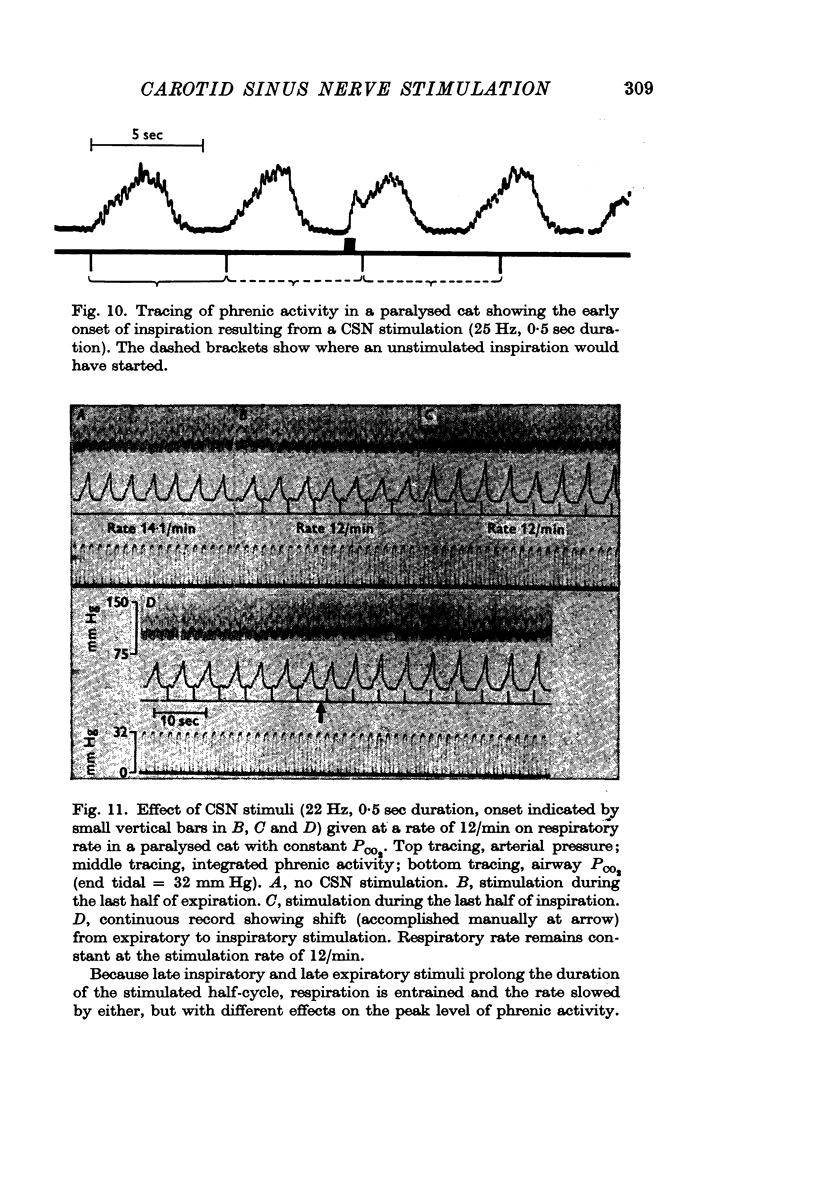
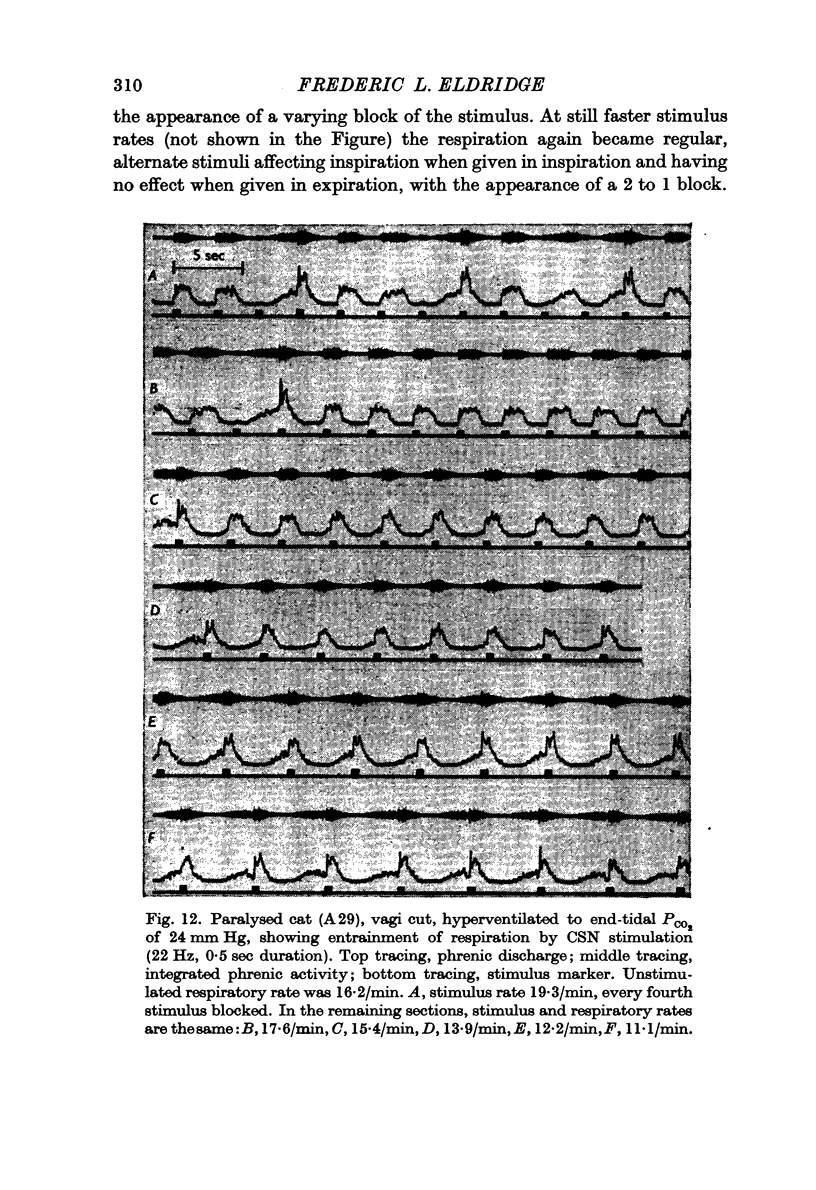
3. Stimuli in expiration led to a prolongation of expiration. Stimuli in late inspiration caused a prolongation of both inspiration and expiration. Because of these effects, the respiratory rate could be changed by stimulation; in some instances entrainment of respiration by the intermittent carotid sinus nerve stimuli occurred.
4. The findings are attributable to modulation of incoming carotid sinus nerve information by the central respiratory neurones, which use primarily that which arrives during inspiration. They show a possible mechanism by which oscillating signals may have a different effect than their mean level would indicate.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band D. M., Cameron I. R., Semple S. J. Oscillations in arterial pH with breathing in the cat. J Appl Physiol. 1969 Mar;26(3):261–267. doi: 10.1152/jappl.1969.26.3.261. [DOI] [PubMed] [Google Scholar]
- Band D. M., Cameron I. R., Semple S. J. The effect on respiration of abrupt changes in carotid artery pH and PCO2 in the cat. J Physiol. 1970 Dec;211(2):479–494. doi: 10.1113/jphysiol.1970.sp009288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N. K., Cunningham D. J., Goode R. C., Howson M. G., Lloyd B. B. Hypoxia, ventilation, PCO2 and exercise. Respir Physiol. 1970 Jun;9(3):329–347. doi: 10.1016/0034-5687(70)90090-3. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. An analysis of the inhibition of phrenic motoneurones which occurs on stimulation of some cranial nerve afferents. J Physiol. 1970 Aug;209(2):375–393. doi: 10.1113/jphysiol.1970.sp009170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., Torrance R. W. Chemoreceptor effects in the respiratory cycle. J Physiol. 1967 Apr;189(2):59P–61P. [PubMed] [Google Scholar]
- Cunningham D. J., Elliott D. H., Lloyd B. B., Miller J. P., Young J. M. A comparison of the effects of oscillating and steady alveolar partial pressures of oxygen and carbon dioxide on the pulmonary ventilation. J Physiol. 1965 Aug;179(3):498–508. doi: 10.1113/jphysiol.1965.sp007676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS A. B., BRITT A. G., FENN W. O. Alveolar CO2 during the respiratory cycle. J Appl Physiol. 1952 Jan;4(7):535–548. doi: 10.1152/jappl.1952.4.7.535. [DOI] [PubMed] [Google Scholar]
- DUTTON R. E., CHERNICK V., MOSES H., BROMBERGER-BARNEA B., PERMUTT S., RILEY R. L. VENTILATORY RESPONSE TO INTERMITTENT INSPIRED CARBON DIOXIDE. J Appl Physiol. 1964 Sep;19:931–936. doi: 10.1152/jappl.1964.19.5.931. [DOI] [PubMed] [Google Scholar]
- Dutton R. E., Fitzgerald R. S., Gross N. Ventilatory response to square-wave forcing of carbon dioxide at the carotid bodies. Respir Physiol. 1968 Jan;4(1):101–108. doi: 10.1016/0034-5687(68)90011-x. [DOI] [PubMed] [Google Scholar]
- Dutton R. E., Hodson W. A., Davies D. G., Chernick V. Ventilatory adaptation to a step change in PCO2 at the caotid bodies. J Appl Physiol. 1967 Aug;23(2):195–202. doi: 10.1152/jappl.1967.23.2.195. [DOI] [PubMed] [Google Scholar]
- Dutton R. E., Hodson W. A., Davies D. G., Fenner A. Effect of the rate of rise of carotid body PCO2 on the time course of ventilation. Respir Physiol. 1967 Dec;3(3):367–379. doi: 10.1016/0034-5687(67)90065-5. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971 Aug;221(2):535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- FENN W. O., CRAIG A. B., Jr EFFECT OF CO2 ON RESPIRATION USING A NEW METHOD OF ADMINISTERING CO2. J Appl Physiol. 1963 Sep;18:1023–1024. doi: 10.1152/jappl.1963.18.5.1023. [DOI] [PubMed] [Google Scholar]
- Fenner A., Berndt J. Ventilatory response to oscillating and non-oscillating PaCO2 in the anesthetized cat. Pflugers Arch. 1970;318(2):108–116. doi: 10.1007/BF00586490. [DOI] [PubMed] [Google Scholar]
- Fenner A., Jansson E. H., Avery M. E. Enhancement of the ventilatory response to carbon dioxide by tube breathing. Respir Physiol. 1968 Jan;4(1):91–100. doi: 10.1016/0034-5687(68)90010-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Leitner L. M., Liaubet M. J. Carotid chemoreceptor response to intermittent or sustained stimulation in the cat. Respir Physiol. 1969 Apr;6(3):395–402. doi: 10.1016/0034-5687(69)90037-1. [DOI] [PubMed] [Google Scholar]
- Goode R. C., Brown E. B., Jr, Howson M. G., Cunningham D. J. Respiratory effects of breathing down a tube. Respir Physiol. 1969 Apr;6(3):343–359. doi: 10.1016/0034-5687(69)90033-4. [DOI] [PubMed] [Google Scholar]
- HONDA Y., UEDA M. Fluctuations of arterial pH associated with the respiratory cycle in dogs. Jpn J Physiol. 1961 Jun 15;11:223–228. doi: 10.2170/jjphysiol.11.223. [DOI] [PubMed] [Google Scholar]
- HORNBEIN T. F., GRIFFO Z. J., ROOS A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol. 1961 Nov;24:561–568. doi: 10.1152/jn.1961.24.6.561. [DOI] [PubMed] [Google Scholar]
- Howard P., Bromberger-Barnea B., Fitzgerald R. S., Bane H. N. Ventilatory responses to peripheral nerve stimulation at different times in the respiratory cycle. Respir Physiol. 1969 Oct;7(3):389–398. doi: 10.1016/0034-5687(69)90022-x. [DOI] [PubMed] [Google Scholar]
- Lamb T. W. Ventilatory responses to intravenous and inspired carbon dioxide in anesthetized cats. Respir Physiol. 1966 Dec;2(1):99–104. doi: 10.1016/0034-5687(66)90041-7. [DOI] [PubMed] [Google Scholar]
- NEIL E., REDWOOD C. R. M., SCHWEITZER A. Pressor responses to electrical stimulation of the carotid sinus nerve in cats. J Physiol. 1949 Sep;109(3-4):259–271. doi: 10.1113/jphysiol.1949.sp004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves M. J. Fluctuations of arterial oxygen tension which have the same period as respiration. Respir Physiol. 1966;1(3):281–296. doi: 10.1016/0034-5687(66)90047-8. [DOI] [PubMed] [Google Scholar]
- Weiss H. R., Salzano J. Formation of whole number ratios of heart rate and breathing frequency. J Appl Physiol. 1970 Sep;29(3):350–354. doi: 10.1152/jappl.1970.29.3.350. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO W. S., EDWARDS M. W., Jr Homeostasis of carbon dioxide during intravenous infusion of carbon dioxide. J Appl Physiol. 1960 Sep;15:807–818. doi: 10.1152/jappl.1960.15.5.807. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO W. S. Mathematical analysis of the time course of alveolar carbon dioxide. J Appl Physiol. 1960 Mar;15:215–219. doi: 10.1152/jappl.1960.15.2.215. [DOI] [PubMed] [Google Scholar]







