Abstract
1. The respiratory response, measured directly as tidal volume or indirectly by using integrated peak phrenic activity, to brief intermittent chemical stimulation or depression of the carotid body was determined in anaesthetized cats. Recordings of carotid sinus nerve impulses allowed precise timing of the stimulus.
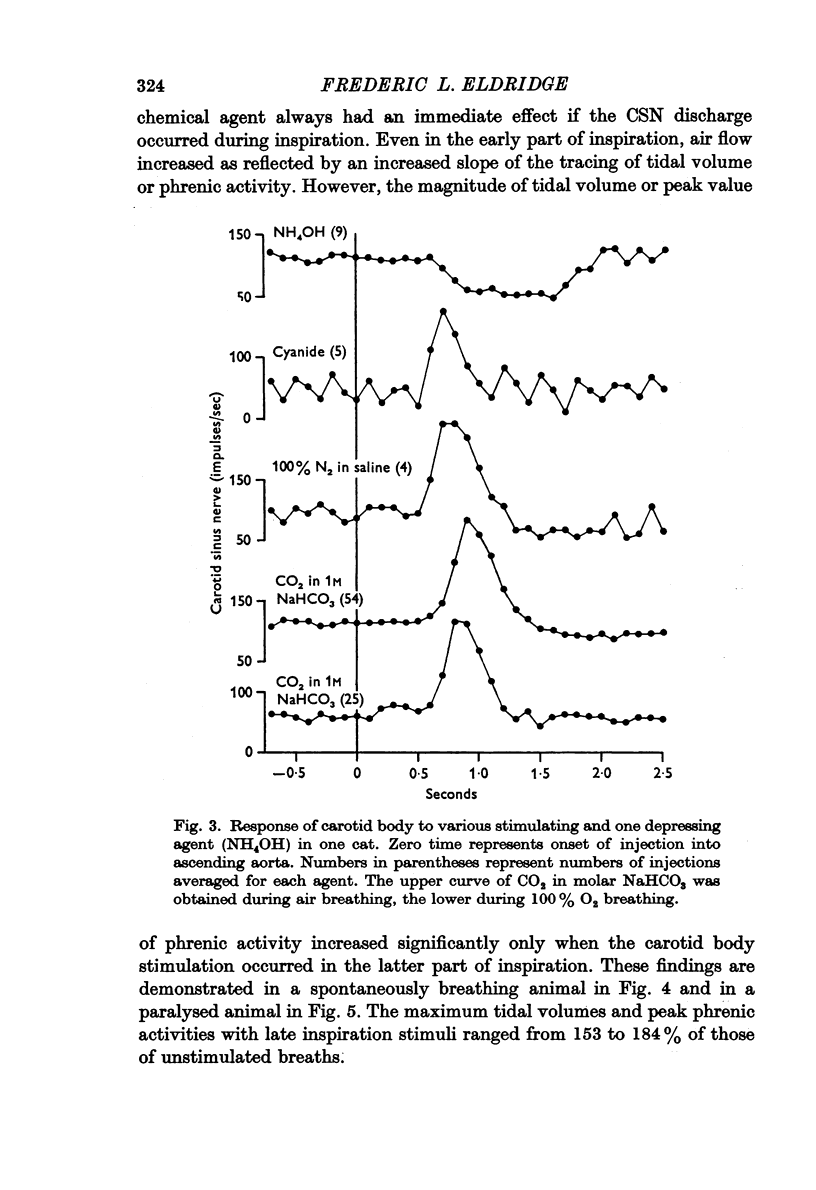
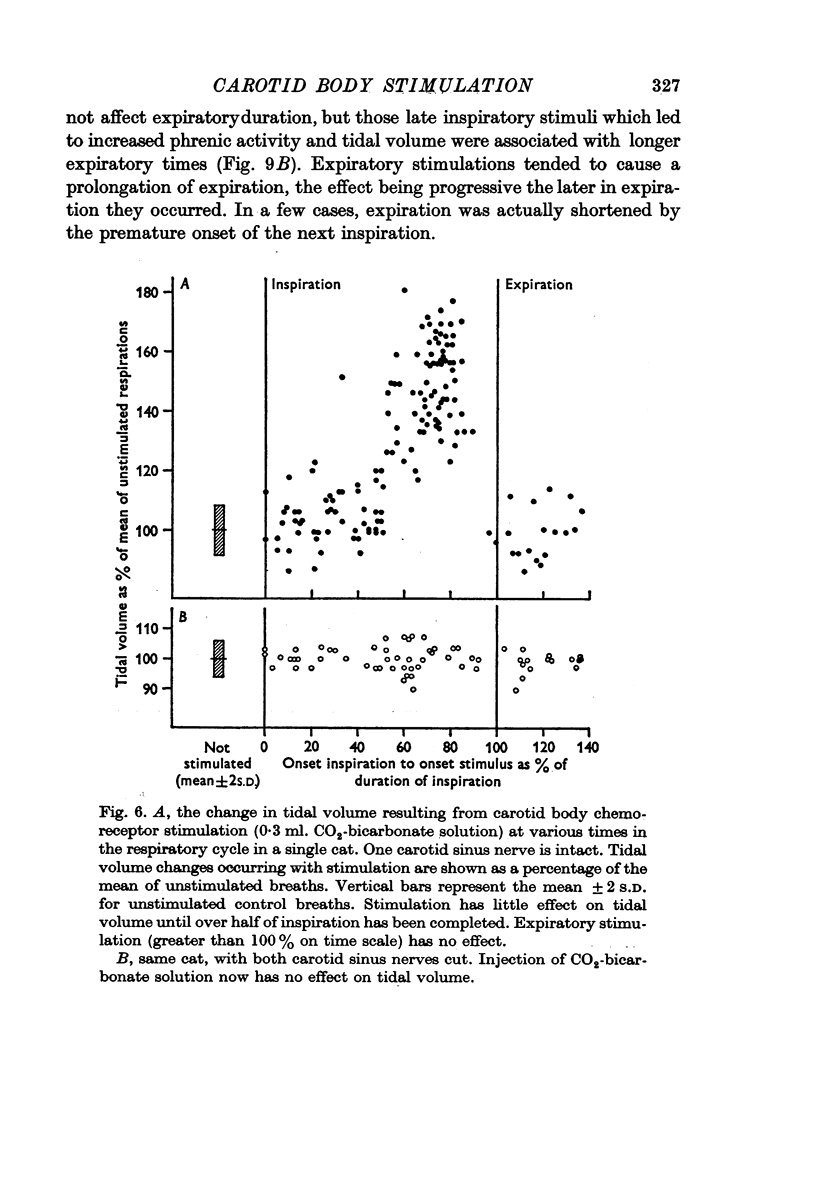
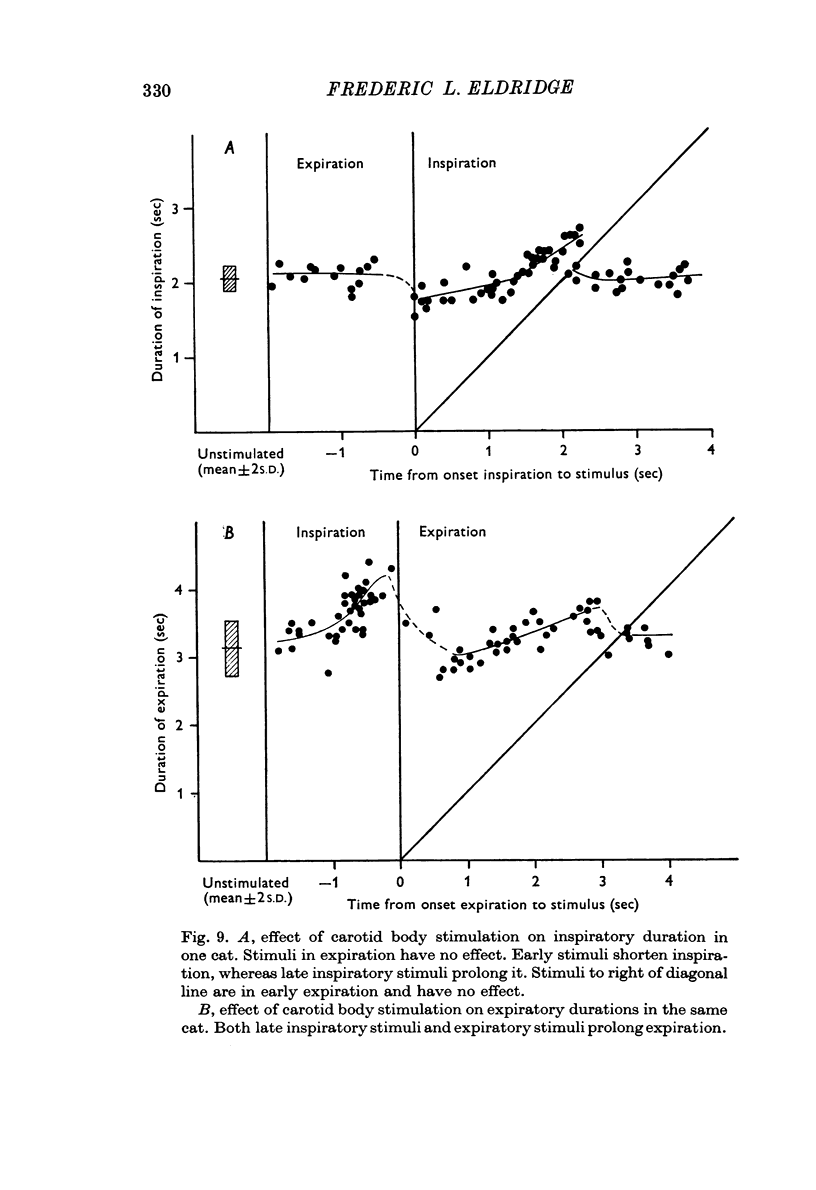
2. Stimulation of the carotid body had a rapid effect on air flow, tidal volume and phrenic discharge rate only if given during inspiration. Increases in tidal volume and peak phrenic discharge occurred only if stimulation was applied during the last half of inspiration. Stimulation during expiration had no effect on the form or magnitude of subsequent breaths.
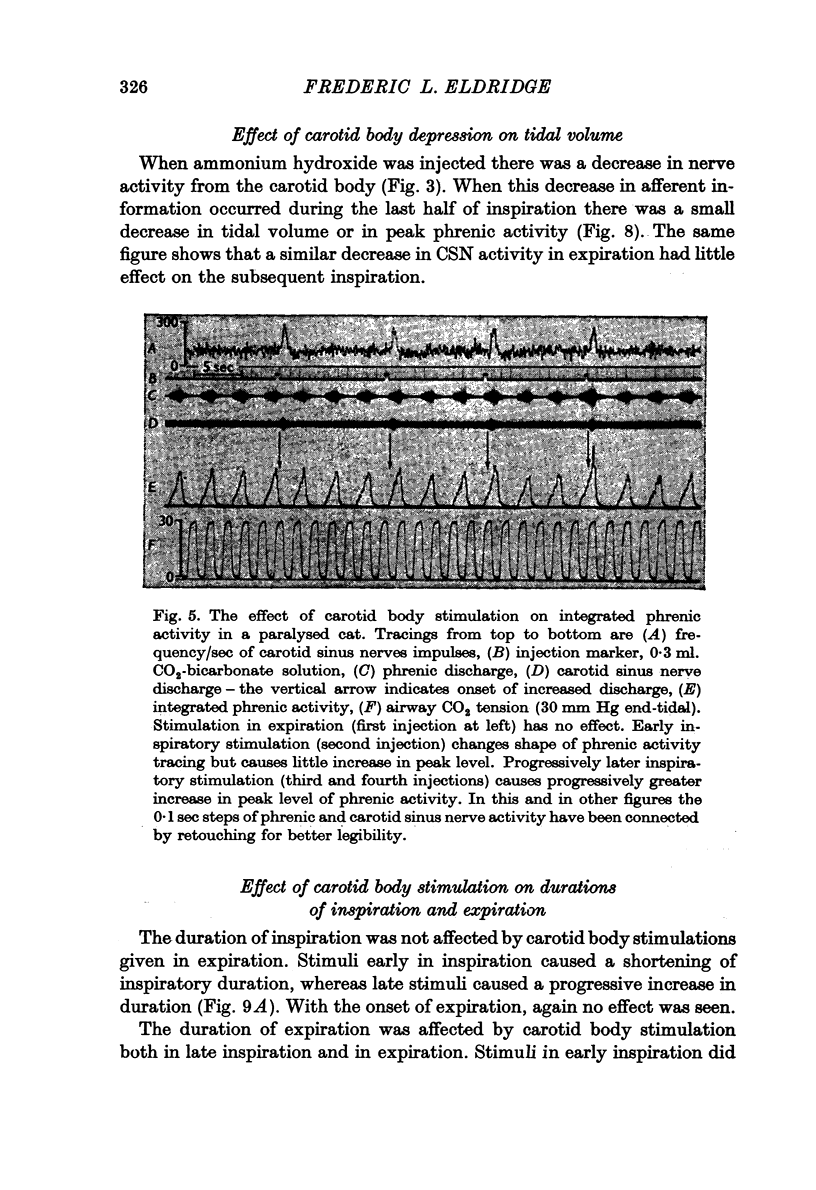
3. Depression of the carotid body by NH4OH led to decreased tidal volume and phrenic discharge if it occurred during inspiration but had no effect if it occurred during expiration.
4. Stimuli in expiration led to a prolongation of expiration. Stimuli in late inspiration caused prolongation of both inspiration and expiration.
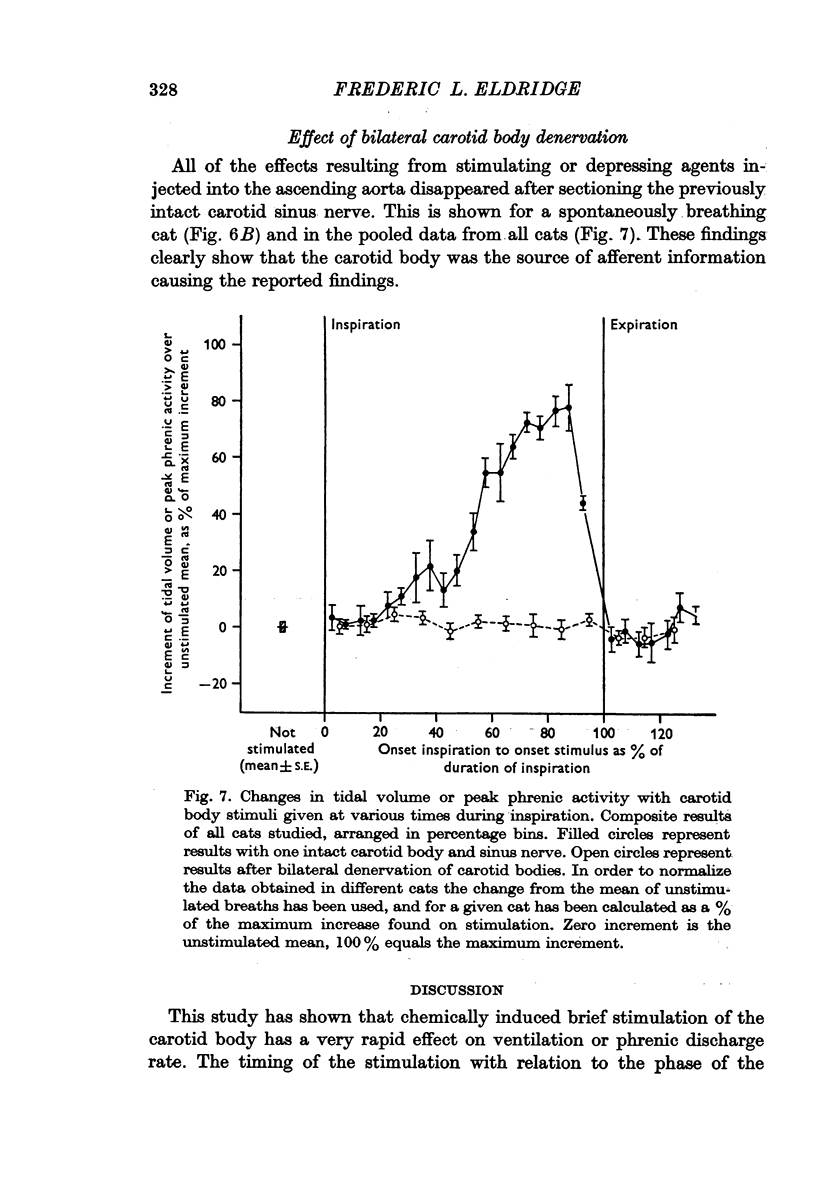
5. All of the effects noted were eliminated by bilateral carotid body denervation.
6. The findings are similar to those following electrical stimulation of the carotid sinus nerve and are attributable to modulation of carotid body signals by the central respiratory neurones.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS B. D., SALMOIRAGHI G. C. Repetitive firing of respiratory neurones during their burst activity. J Neurophysiol. 1960 Jan;23:27–46. doi: 10.1152/jn.1960.23.1.27. [DOI] [PubMed] [Google Scholar]
- Band D. M., Cameron I. R., Semple S. J. The effect on respiration of abrupt changes in carotid artery pH and PCO2 in the cat. J Physiol. 1970 Dec;211(2):479–494. doi: 10.1113/jphysiol.1970.sp009288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., Torrance R. W. Chemoreceptor effects in the respiratory cycle. J Physiol. 1967 Apr;189(2):59P–61P. [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971 Aug;221(2):535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Gross N., Dutton R. E. Ventilatory responses to transient acidic and hypercapnic vertebral artery infusions. Respir Physiol. 1968 May;4(3):387–395. doi: 10.1016/0034-5687(68)90043-1. [DOI] [PubMed] [Google Scholar]
- Howard P., Bromberger-Barnea B., Fitzgerald R. S., Bane H. N. Ventilatory responses to peripheral nerve stimulation at different times in the respiratory cycle. Respir Physiol. 1969 Oct;7(3):389–398. doi: 10.1016/0034-5687(69)90022-x. [DOI] [PubMed] [Google Scholar]
- Leitner L. M., Pagès B., Puccinelli R., Dejours P. Etude simultanée de la ventilation et des décharges des chémorécepteurs du glomus carotidien chez le chat. II. Au cours d'inhalations brèves d'anhydride carbonique. Arch Int Pharmacodyn Ther. 1965 Apr;154(2):427–433. [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., von BAUMGARTEN Intracellular potentials from respiratory neurones in brain-stem of cat and mechanism of rhythmic respiration. J Neurophysiol. 1961 Mar;24:203–218. doi: 10.1152/jn.1961.24.2.203. [DOI] [PubMed] [Google Scholar]
- Tenney S. M., Brooks J. G., 3rd Carotid bodies, stimulus interaction, and ventilatory control in unanesthetized goats. Respir Physiol. 1966;1(2):211–224. doi: 10.1016/0034-5687(66)90018-1. [DOI] [PubMed] [Google Scholar]