Abstract
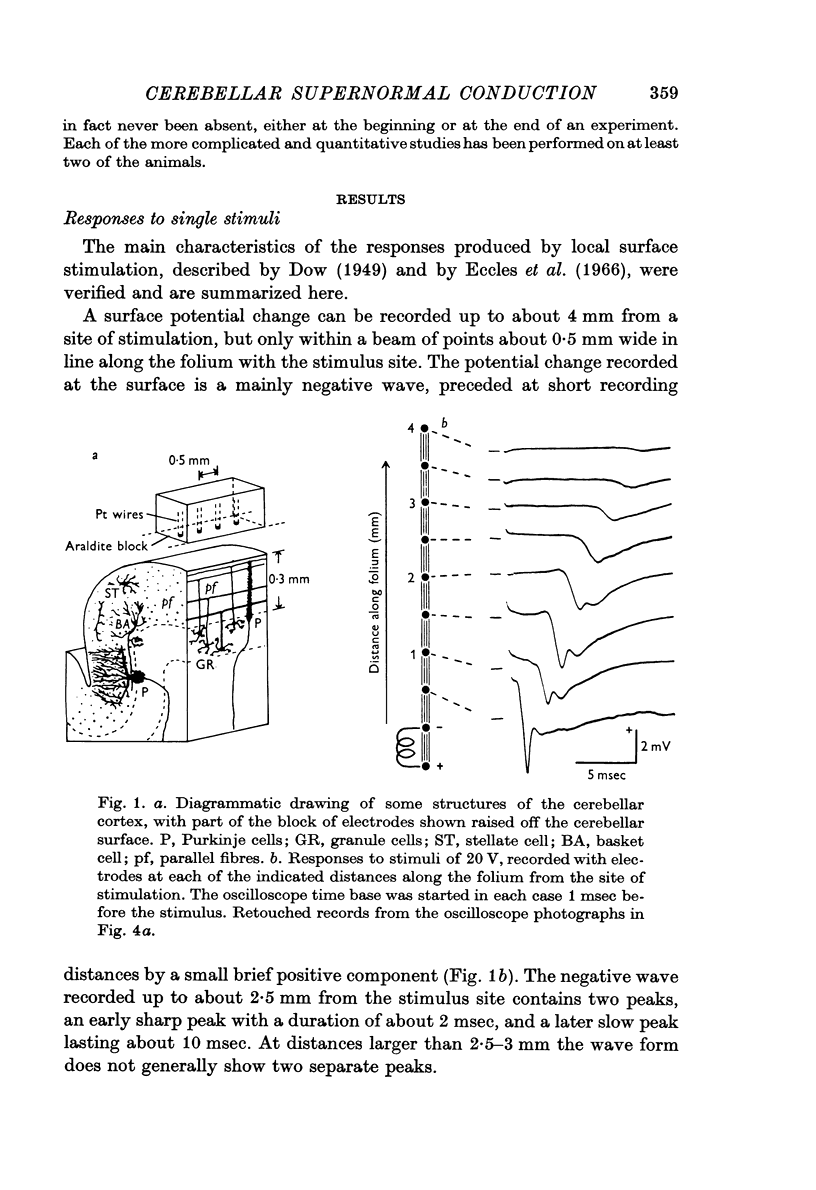
1. The electrical responses produced by stimulation at the surface of the cerebellar cortex have been studied in anaesthetized cats.
2. The propagation of the underlying activity is attributed to parallel fibres, but the mode of generation of the potential changes is less certain.
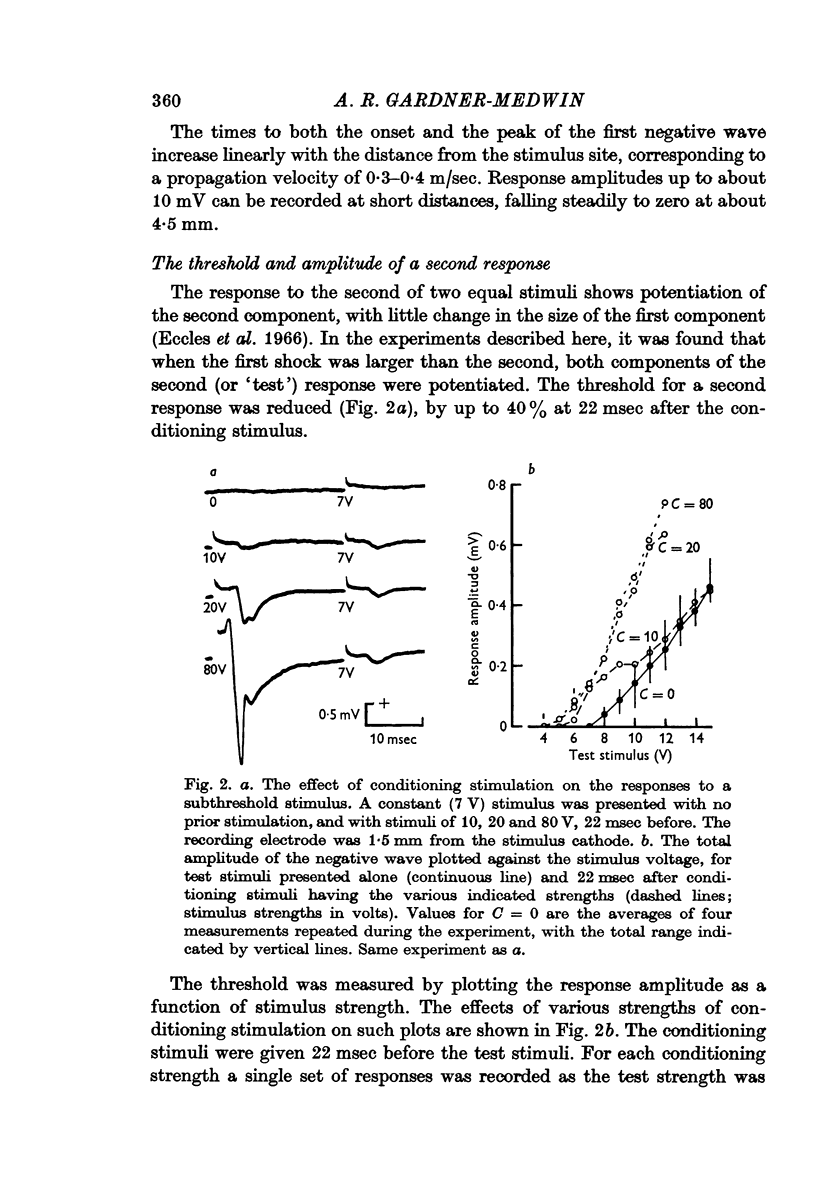
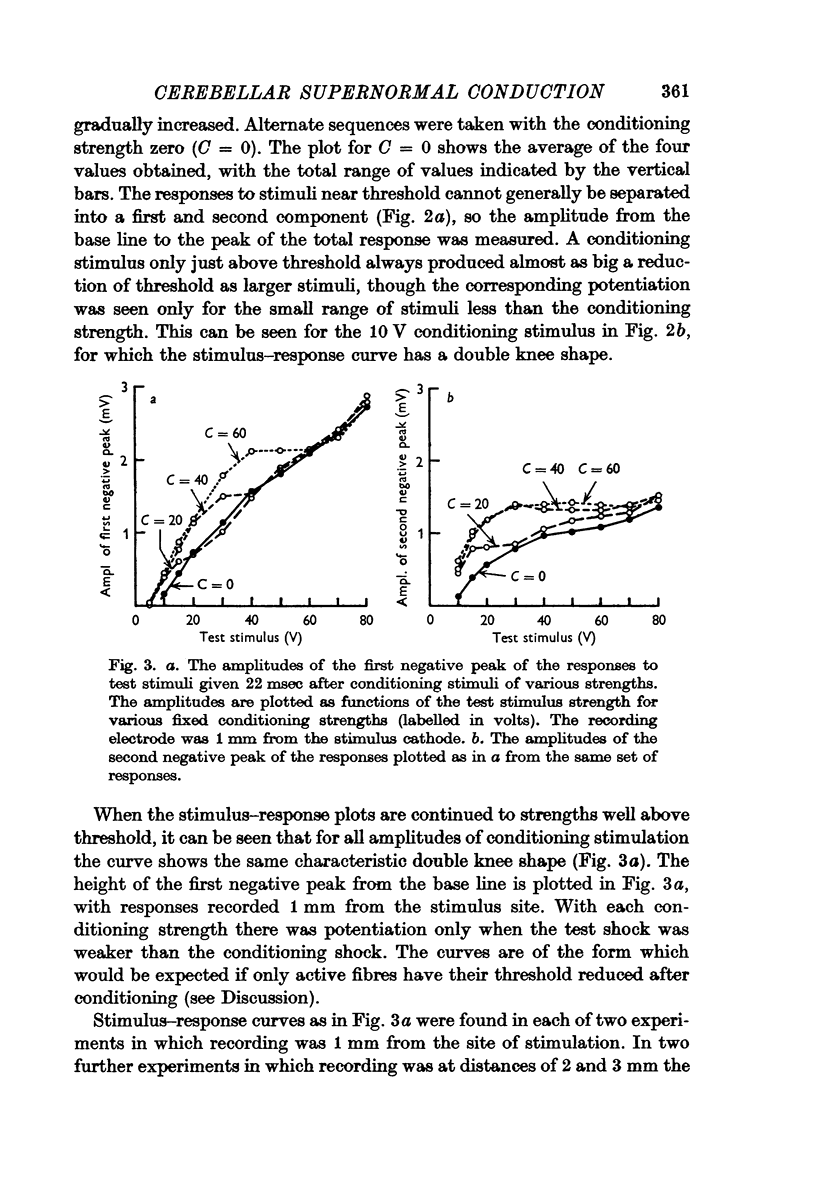
3. The threshold for a second response is up to 40% lower following a conditioning stimulus, and the responses to test stimuli less strong than the conditioning stimulus are correspondingly potentiated.
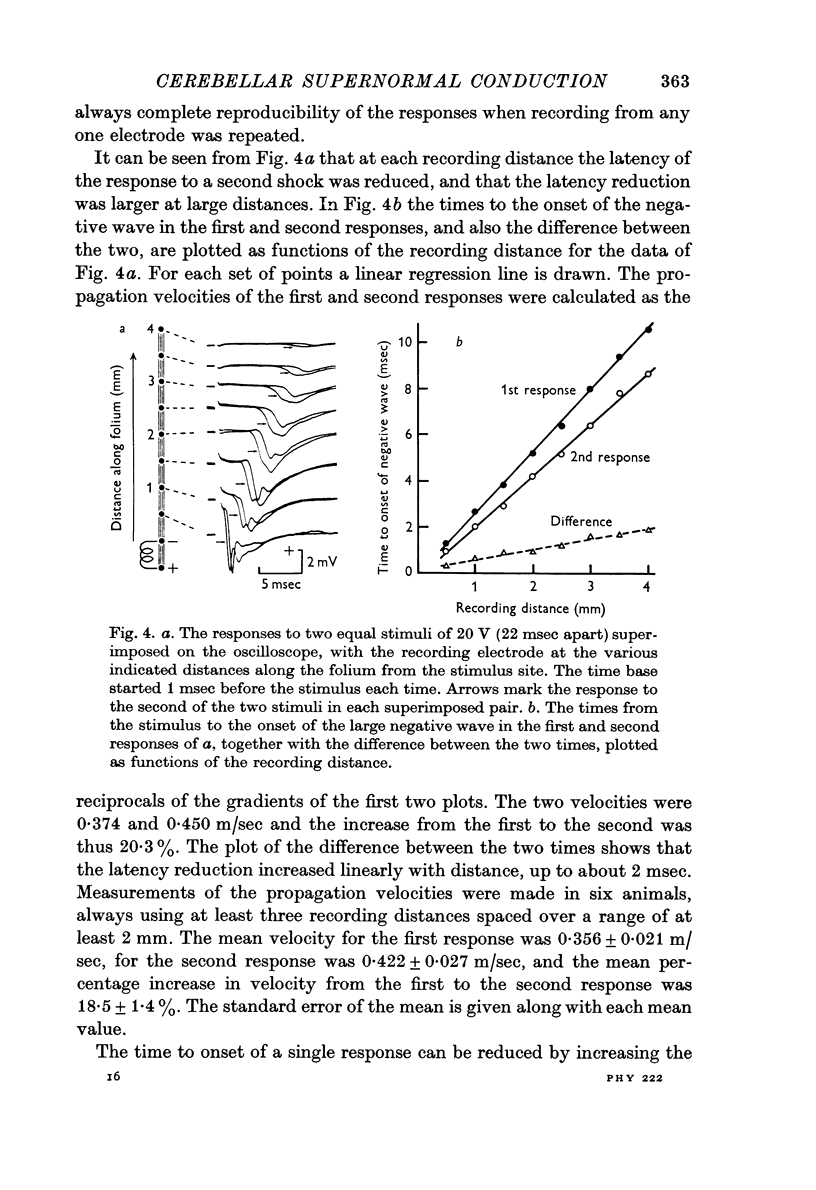
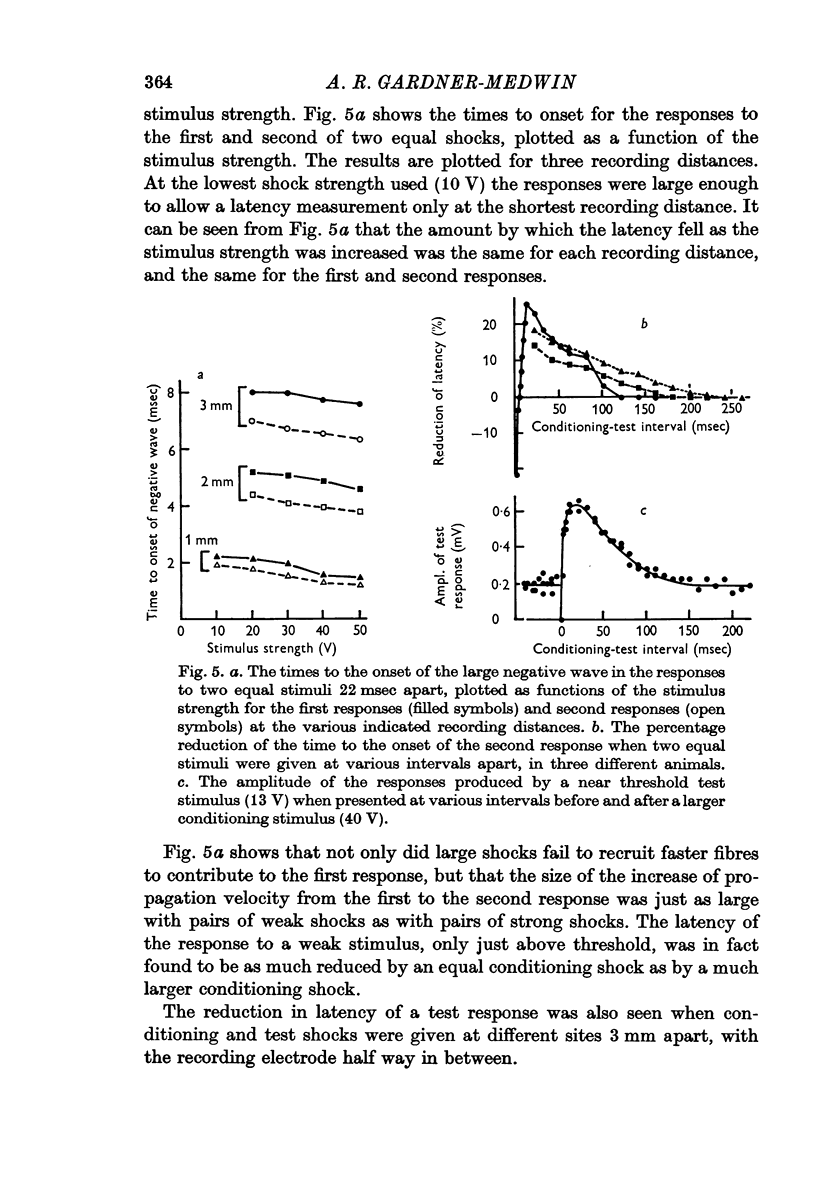
4. A second stimulus given 22 msec after the first produces a response which propagates 15-20% faster along the folium.
5. The reduction of the threshold and the increase of the propagation velocity are as large with small (but above threshold) conditioning shocks as with large shocks.
6. Both after-effects are present from 5 to 100 msec after a conditioning stimulus, with maximal values at about 10-30 msec.
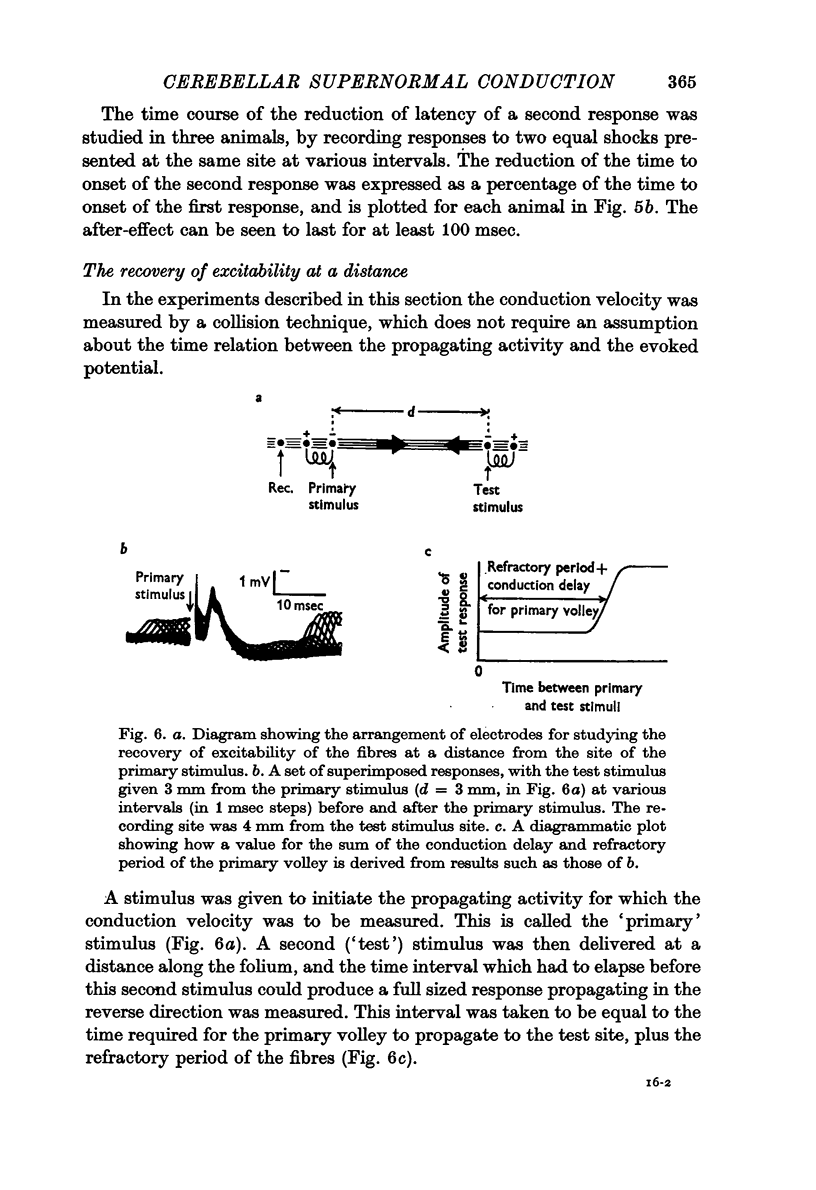
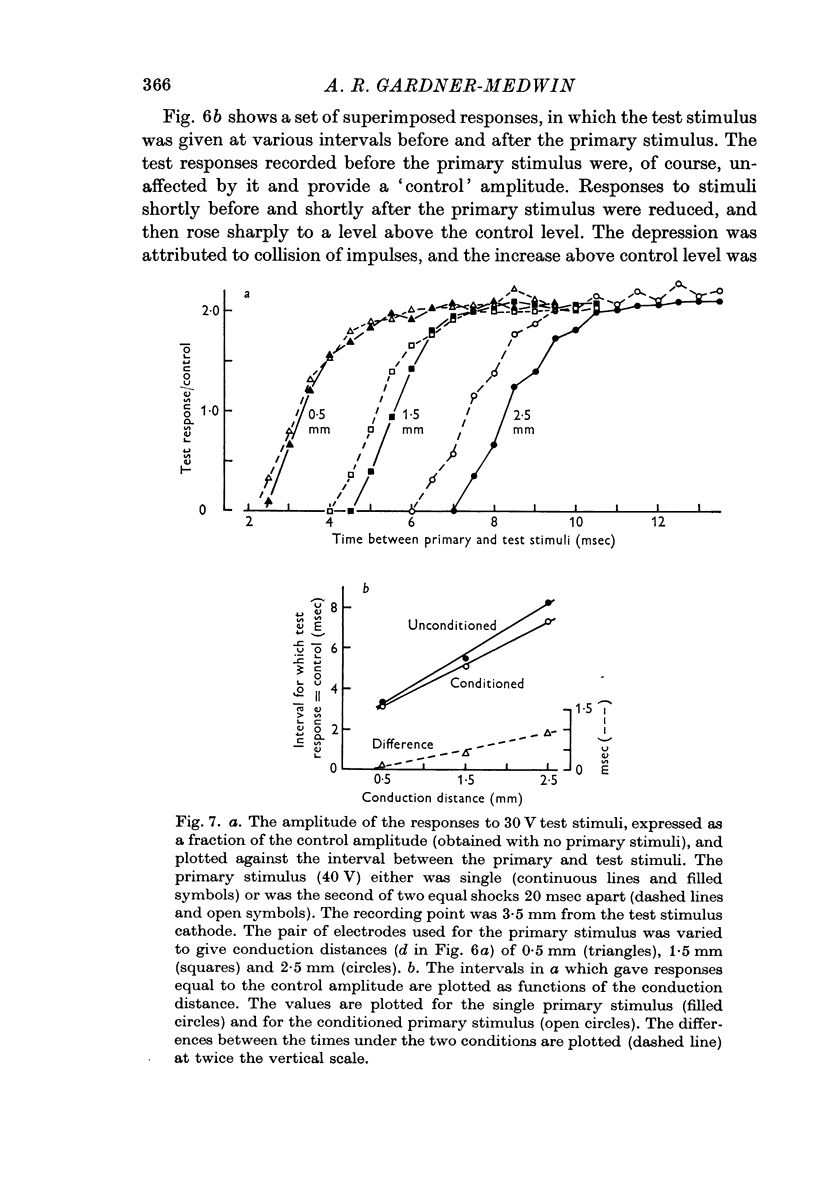
7. The fibre conduction velocity inferred from collision experiments agrees with that inferred from the propagation velocity of the responses, and shows a similar increase after conditioning.
8. A second shock does not recruit a new and faster population of parallel fibres.
9. Under the conditions of electrical stimulation the after-effects are probably restricted to those fibres which are active during conditioning.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLOCK T. H. Facilitation of conduction rate in nerve fibers. J Physiol. 1951 Jun;114(1-2):89–97. doi: 10.1113/jphysiol.1951.sp004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- FOX C. A., BARNARD J. W. A quantitative study of the Purkinje cell dendritic branchlets and their relationship to afferent fibres. J Anat. 1957 Jul;91(3):299–313. [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Hillman D. E., Siegesmund K. A., Dutta C. R. The primate cerebellar cortex: a Golgi and electron microscopic study. Prog Brain Res. 1967;25:174–225. doi: 10.1016/S0079-6123(08)60965-6. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Properties of dorsal root unmedullated fibers on the two sides of the ganglion. J Gen Physiol. 1955 May 20;38(5):709–728. doi: 10.1085/jgp.38.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. A supernormal period after an action potential in parallel fibres of the cerebellum. J Physiol. 1971 Jul;216(2):59P–60P. [PubMed] [Google Scholar]
- Gobel S. Electron microscopical studies of the cerebellar molecular layer. J Ultrastruct Res. 1967 Dec;21(5):430–458. doi: 10.1016/s0022-5320(67)80151-5. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALAY S. L., McGEE-RUSSELL S. M., GORDON S., Jr, GRILLO M. A. Fixation of neural tissues for electron microscopy by perfusion with solutions of osmium tetroxide. J Cell Biol. 1962 Feb;12:385–410. doi: 10.1083/jcb.12.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


