Abstract
1. A new simple method is described for quantitating the adhesiveness of circulating polymorphonuclear leucocytes, or granulocytes, to the walls of blood vessels. The cheek pouch of anaesthetized hamsters or a small part of the mesentery of anaesthetized mice were prepared for continuous microscopic observation of selected venules. Those granulocytes which moved sufficiently slowly to be individually visible were counted for 1 or 2 min periods as they rolled past a selected point on one side of a vessel. The velocity distribution of these cells was determined by analysing films. Films were used also to measure mean blood flow velocity in the venules by observing embolizing platelet thrombi induced by the iontophoretic application of adenosine diphosphate. Emigration of granulocytes into the tissues was quantitated by enumerating them in standard areas of stained histological sections.
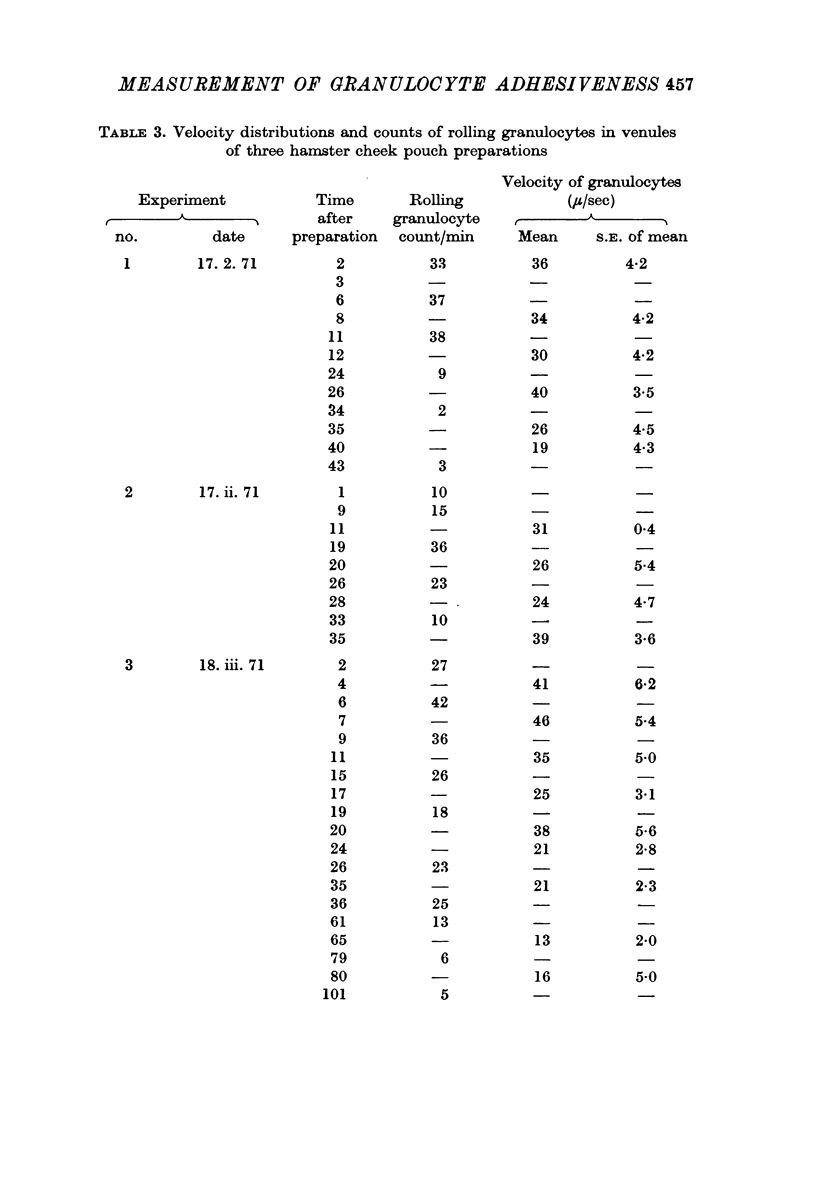
2. In control experiments with hamster cheek pouch venules, the rolling granulocyte count usually passed through a maximum shortly after the preparation was set up and then fell to a low constant value. In mouse mesentery venules the count remained at a low approximately constant value from the beginning for at least 3 hr.
3. The mean velocity of blood flow in the venules was between 900 and 200 μ/sec. All rolling granulocytes moved much more slowly; in hamster cheek pouch venules the mean velocity was about 20 μ/sec and in mouse mesentery venules about 10 μ/sec. Around these means the velocity distribution of individual cells was narrow.
4. Rolling of granulocytes was abolished by superfusing ethylenediamine tetra-acetate (EDTA, 0·1 M) suggesting that the phenomenon depends on calcium or magnesium ions.
5. Agents were applied locally to the observed venules. Human serum albumin, trypsin or histamine in high concentrations did not affect the rolling granulocyte count.
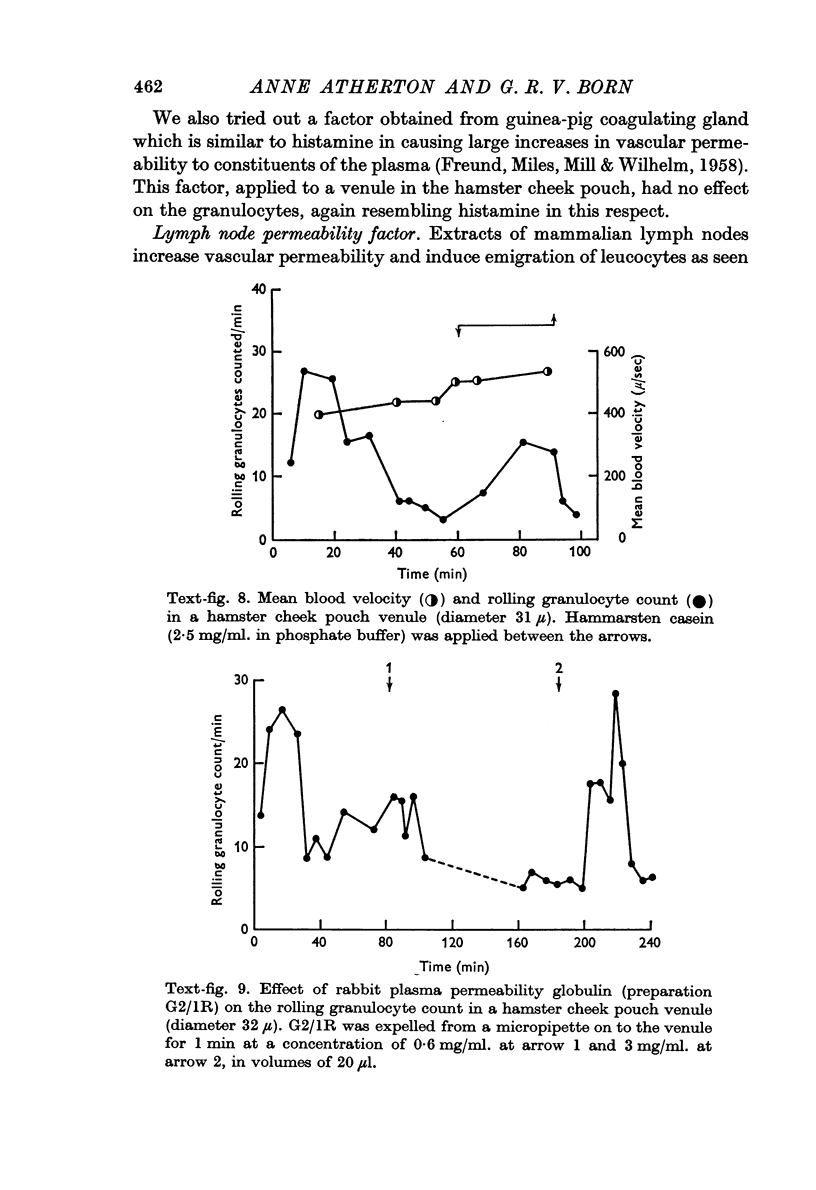
6. The rolling granulocyte count was increased during the application of Hammarsten casein or Escherichia coli culture filtrate which are chemotactic to granulocytes in vitro. These agents did not cause alterations in mean blood flow velocity in the observed venules which might have accounted for the effect on the rolling granulocyte counts. When E. coli culture filtrate was perfused through mouse intestine the increase in rolling granulocyte count in the draining venous blood was proportional to the logarithm of the concentration of filtrate.
7. The rolling granulocyte count was also increased by the local application of plasma globulin permeability factor or lymph node permeability factor.
8. Granulocyte counts in standard histological sections showed no significant increases in control preparations but considerable increases following the application of Hammarsten casein.
Full text
PDF





























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. EFFECTS OF INORGANIC IONS AND OF PLASMA PROTEINS ON THE AGGREGATION OF BLOOD PLATELETS BY ADENOSINE DIPHOSPHATE. J Physiol. 1964 Mar;170:397–414. doi: 10.1113/jphysiol.1964.sp007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begent N., Born G. V. Growth rate in vivo of platelet thrombi, produced by iontophoresis of ADP, as a function of mean blood flow velocity. Nature. 1970 Aug 29;227(5261):926–930. doi: 10.1038/227926a0. [DOI] [PubMed] [Google Scholar]
- FREUND J., MILES A. A., MILL P. J., WILHELM D. L. Vascular permeability factors in the secretion of the guinea pig coagulating gland. Nature. 1958 Jul 19;182(4629):174–175. doi: 10.1038/182174a0. [DOI] [PubMed] [Google Scholar]
- HARRIS H. Chemotaxis of granulocytes. J Pathol Bacteriol. 1953 Jul;66(1):135–146. doi: 10.1002/path.1700660117. [DOI] [PubMed] [Google Scholar]
- HARRIS H. Role of chemotaxis in inflammation. Physiol Rev. 1954 Jul;34(3):529–562. doi: 10.1152/physrev.1954.34.3.529. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Sorkin E. Chemotaxis of leucocytes. Experientia. 1968 Jul 15;24(7):641–652. doi: 10.1007/BF02138287. [DOI] [PubMed] [Google Scholar]
- MARCHESI V. T., FLOREY H. W. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960 Oct;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- MARCHESI V. T., GOWANS J. L. THE MIGRATION OF LYMPHOCYTES THROUGH THE ENDOTHELIUM OF VENULES IN LYMPH NODES: AN ELECTRON MICROSCOPE STUDY. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- SPECTOR W. G., WILLOUGHBY D. A. THE EFFECT OF VASCULAR PERMEABILITY FACTORS ON THE EMIGRATION OF LEUCOCYTES. J Pathol Bacteriol. 1964 Apr;87:341–346. [PubMed] [Google Scholar]
- Thompson P. L., Papadimitriou J. M., Walters M. N. Suppression of leucocytic sticking and emigration by chelation of calcium. J Pathol Bacteriol. 1967 Oct;94(2):389–396. doi: 10.1002/path.1700940219. [DOI] [PubMed] [Google Scholar]
- WILHELM D. L., MILL P. J., SPARROW E. M., MACKAY M. E., MILES A. A. Enzyme-like globulins from serum reproducing the vascular phenomena of inflammation. IV. Activable permeability factor and its inhibitor in the serum of the rat and the rabbit. Br J Exp Pathol. 1958 Jun;39(3):228–250. [PMC free article] [PubMed] [Google Scholar]
- Wood S., Jr, Marzocchi B. E. Motility of granulocytes during wound healing in the rabbit ear chamber. Johns Hopkins Med J. 1968 Jul;123(1):23–28. [PubMed] [Google Scholar]






