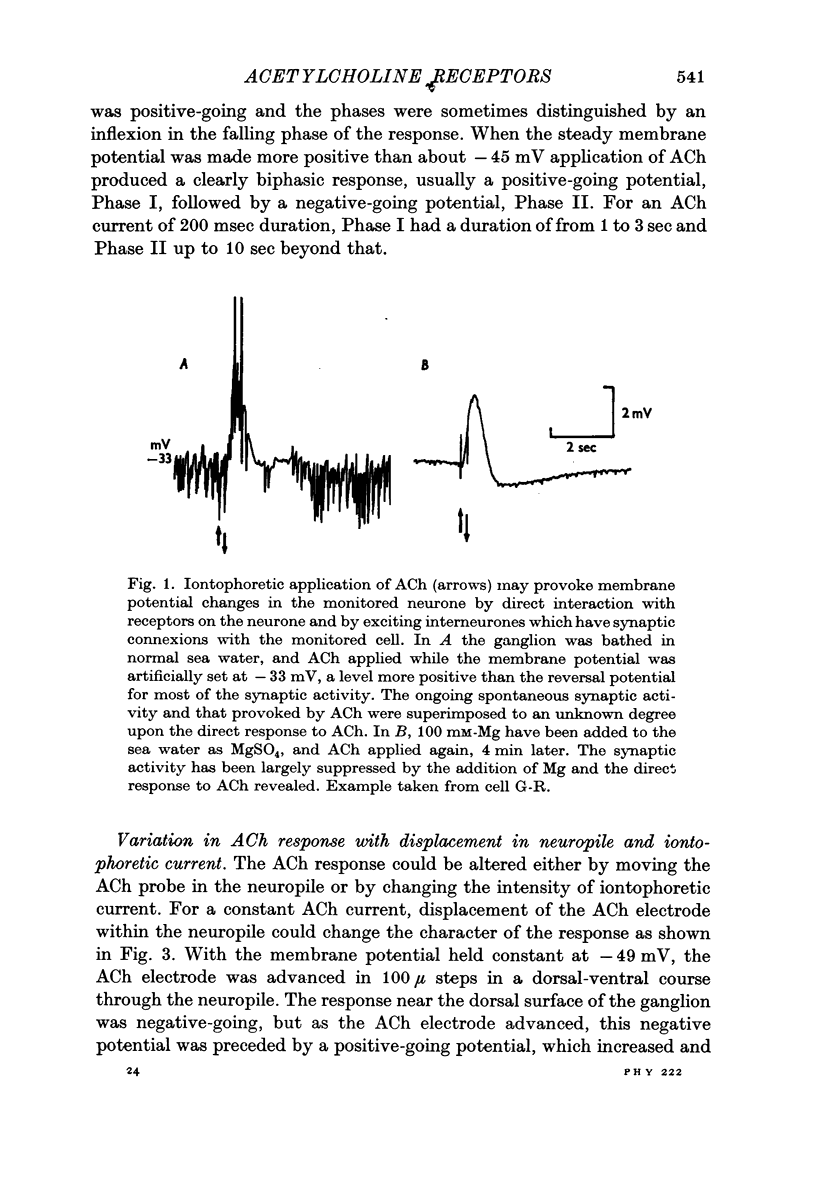
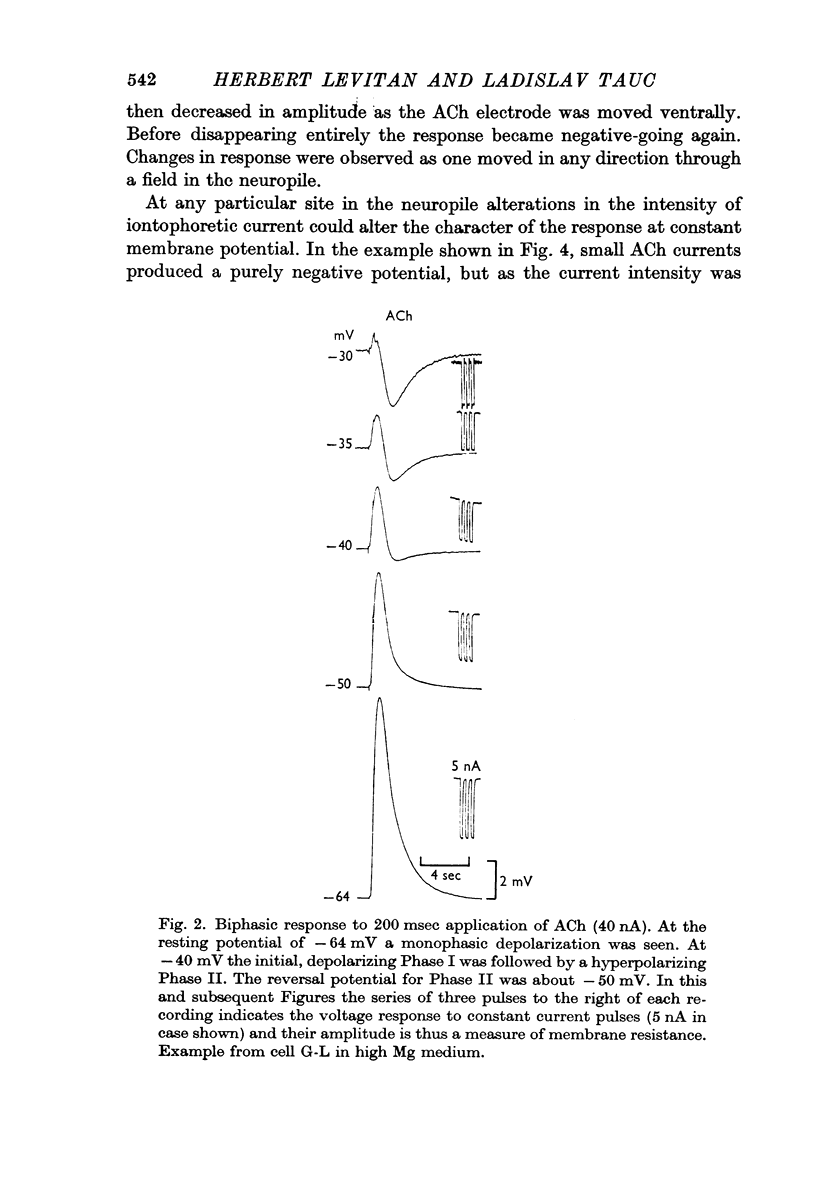
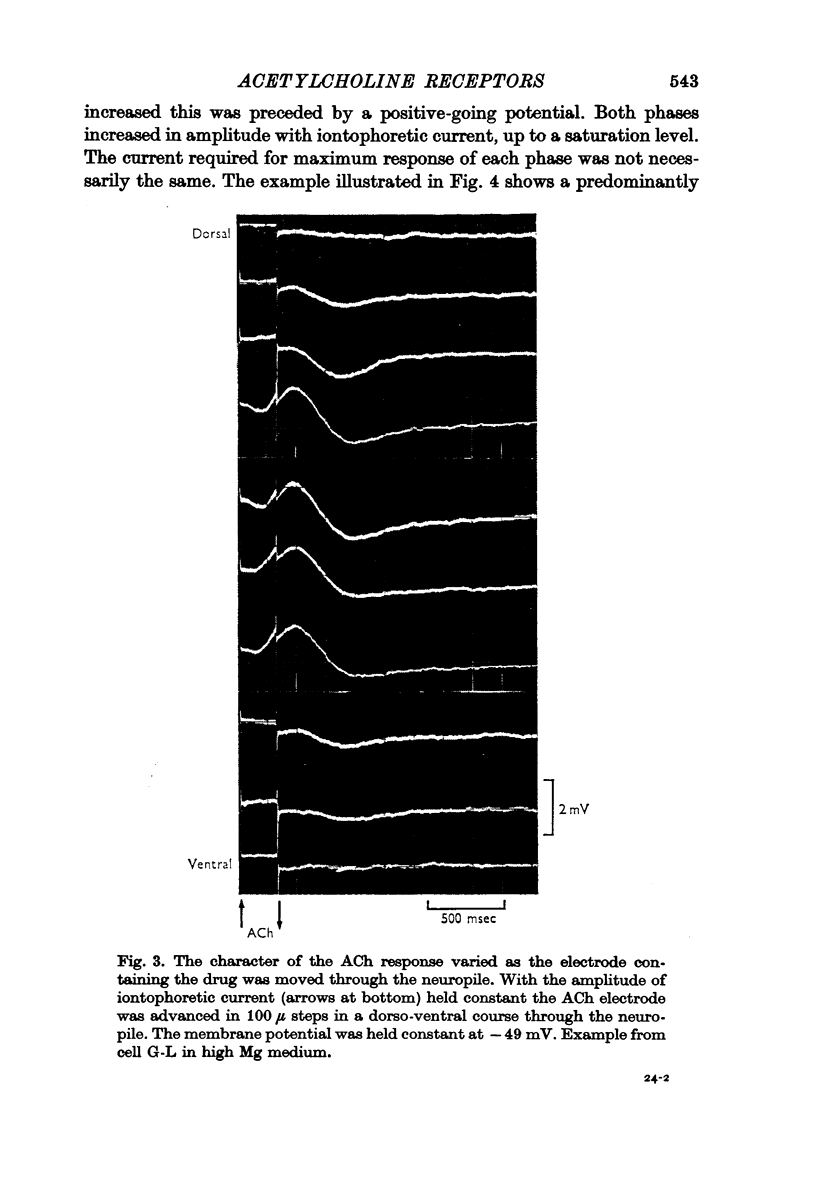
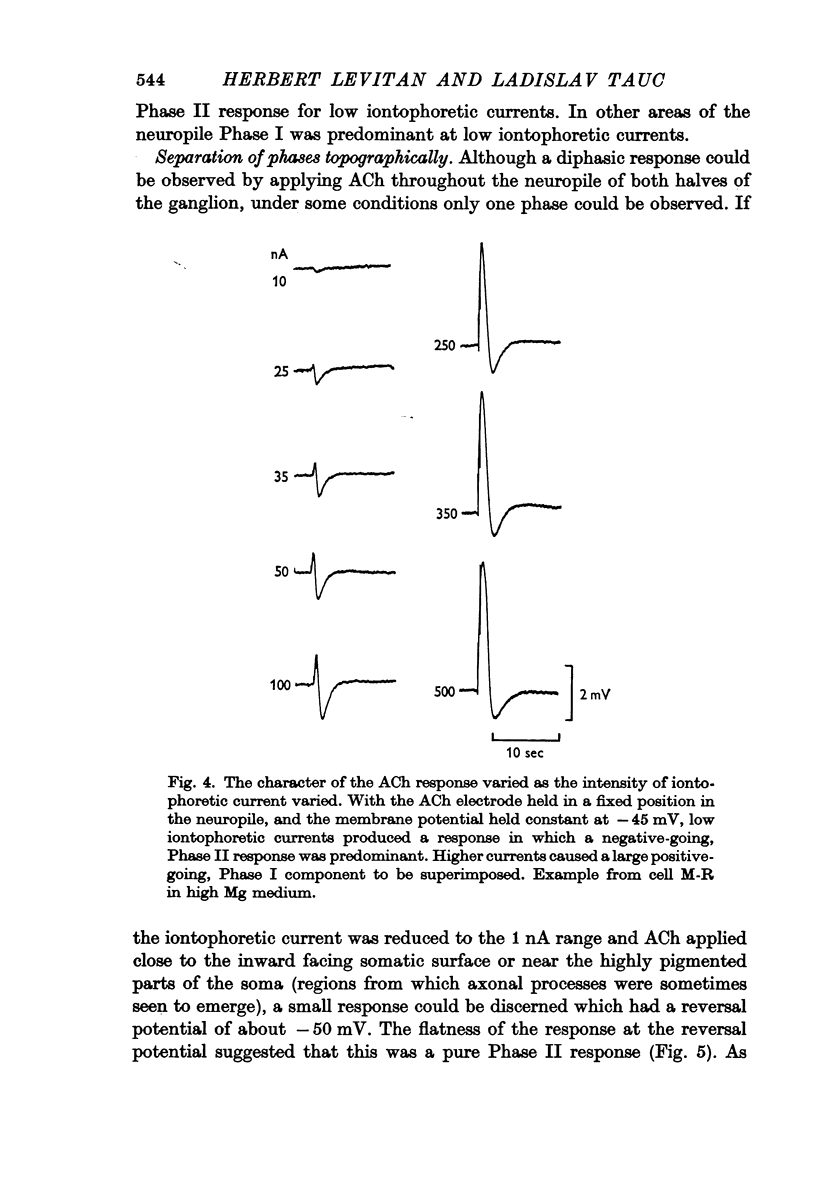
Abstract
1. The iontophoretic application of acetylcholine (ACh) on to identified neurones in the buccal ganglion of the mollusc Navanax produced a biphasic or monophasic membrane potential change which was a function of the current intensity and site of ACh application.
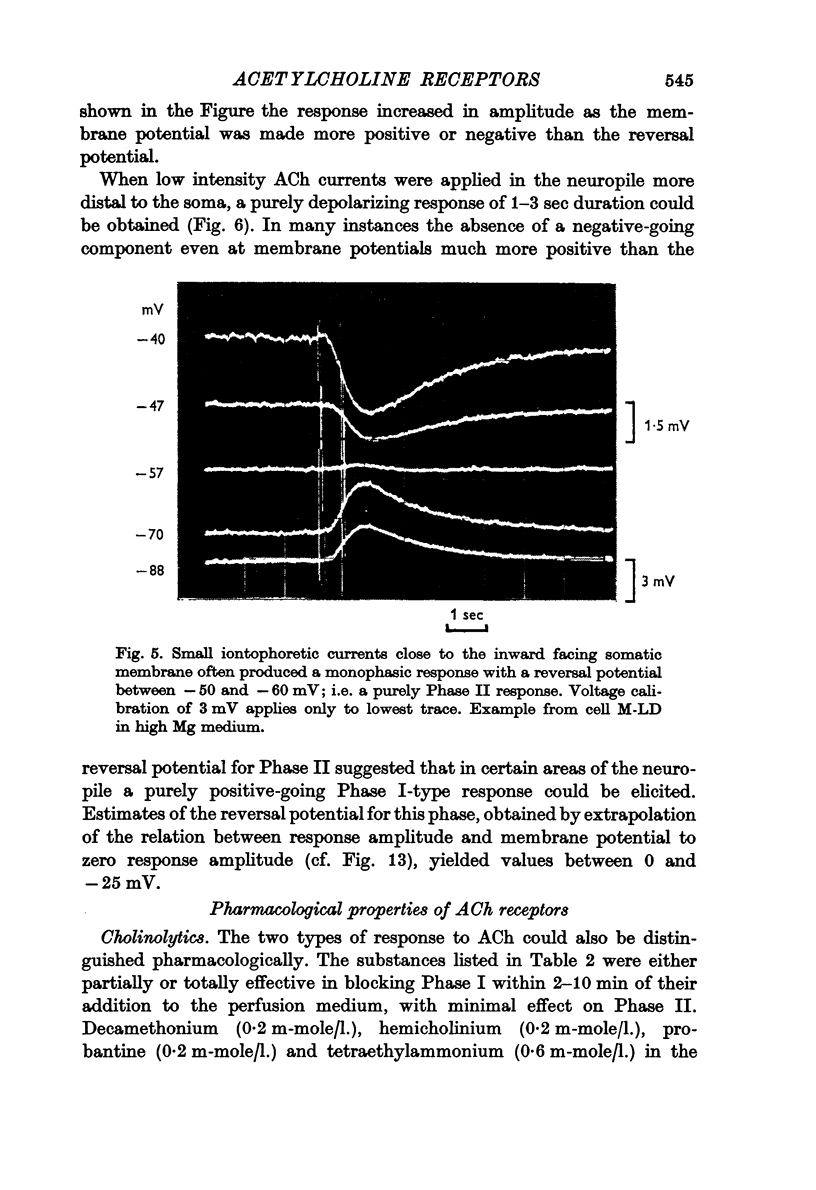
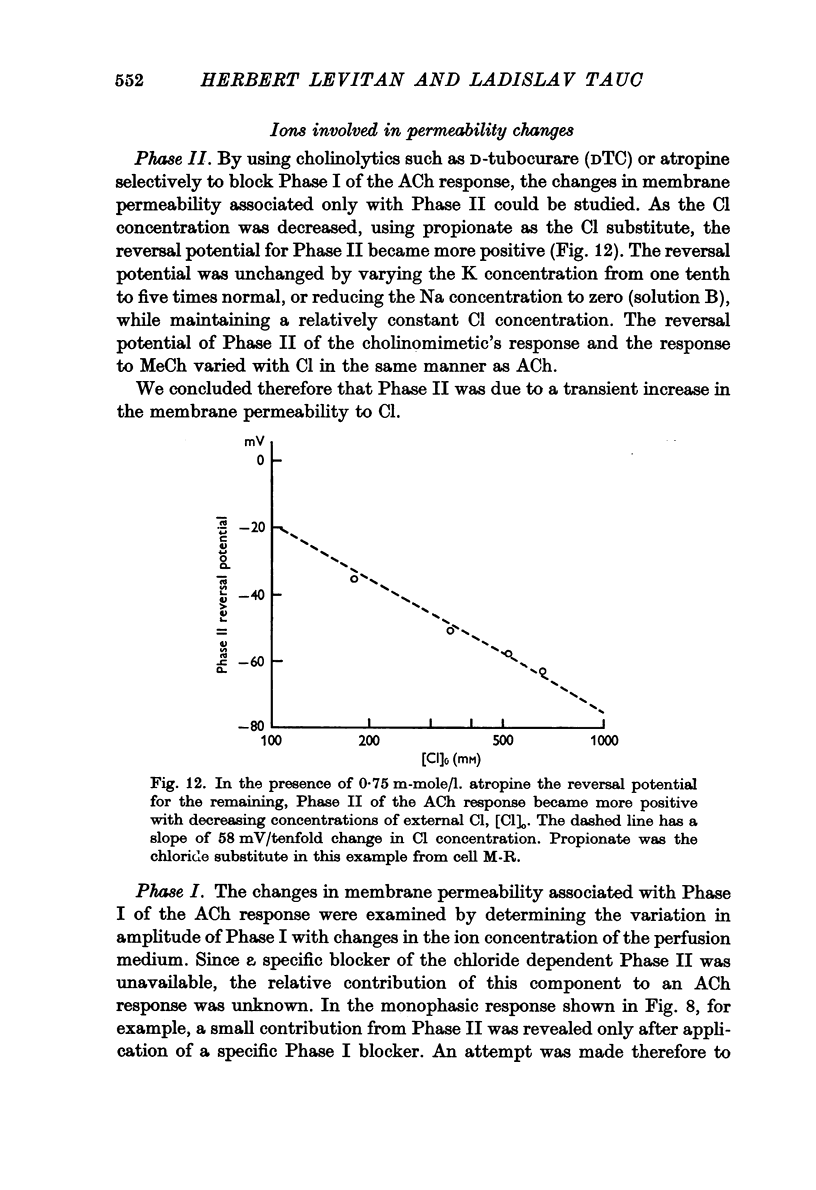
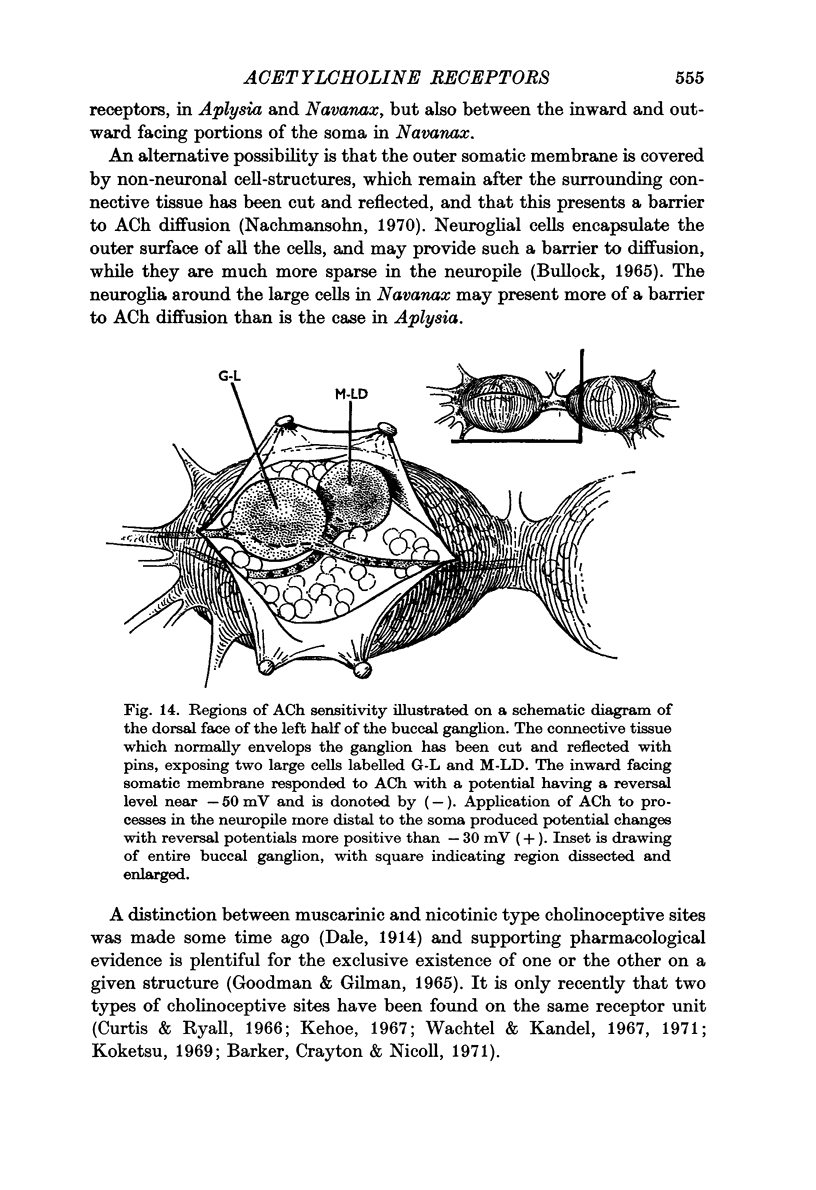
2. Low iontophoretic currents, 200 msec in duration, applied to the somatic surface facing the neuropile, caused a monophasic potential change of 6-10 sec duration, which had a reversal potential of about - 50 mV, varied with changes in the [Cl]o of the bathing medium, and was not blocked by the cholinolytics tested.
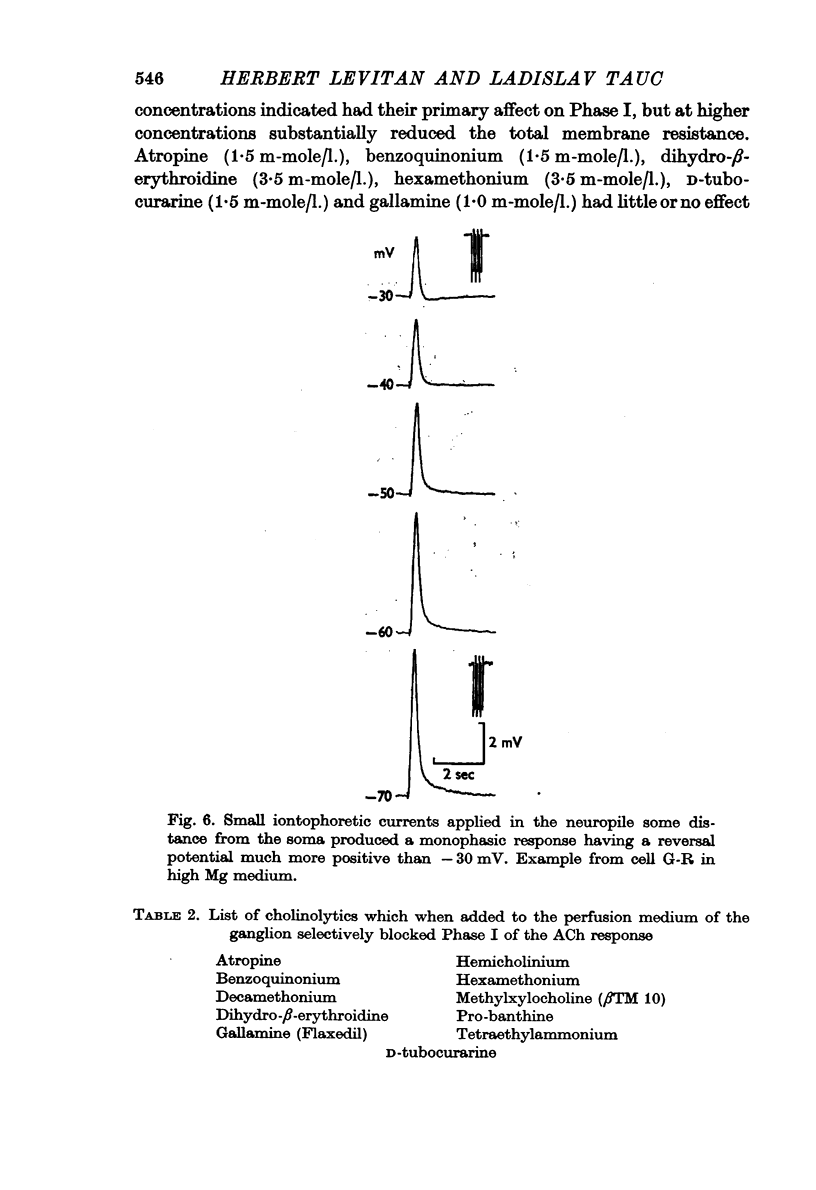
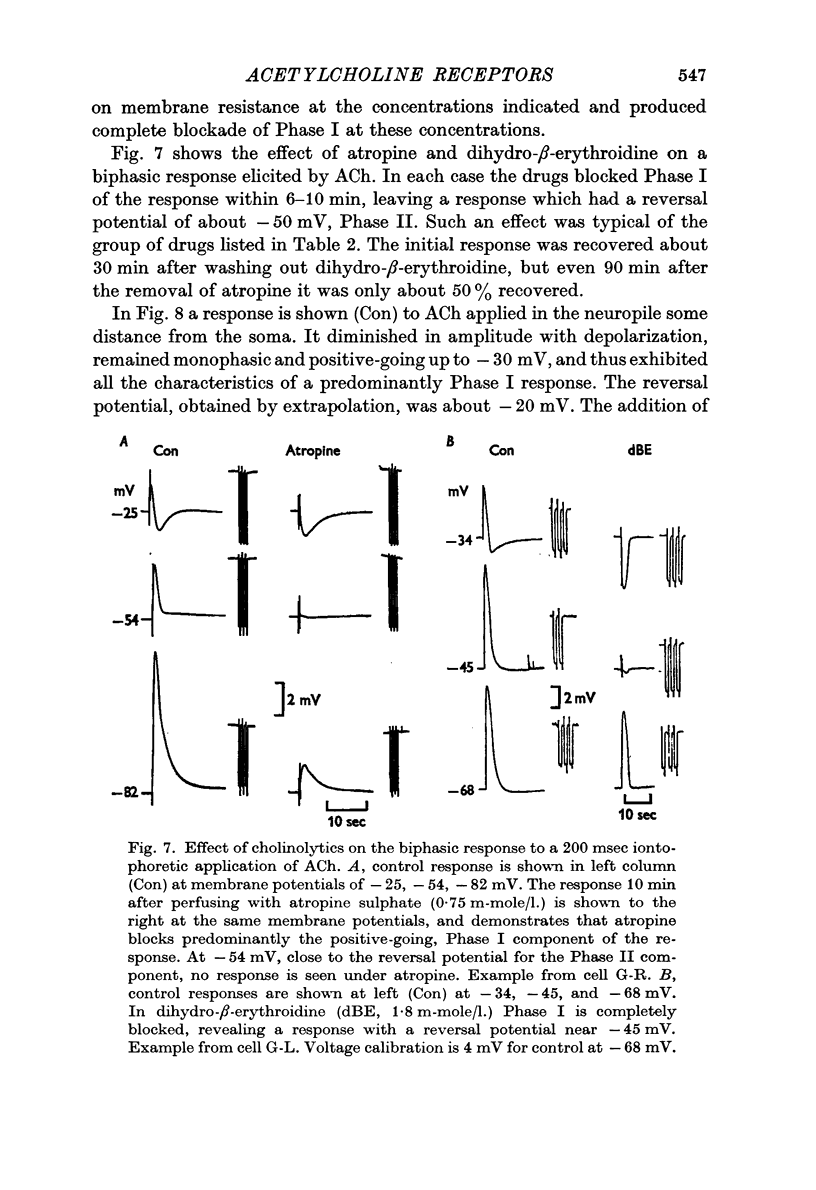
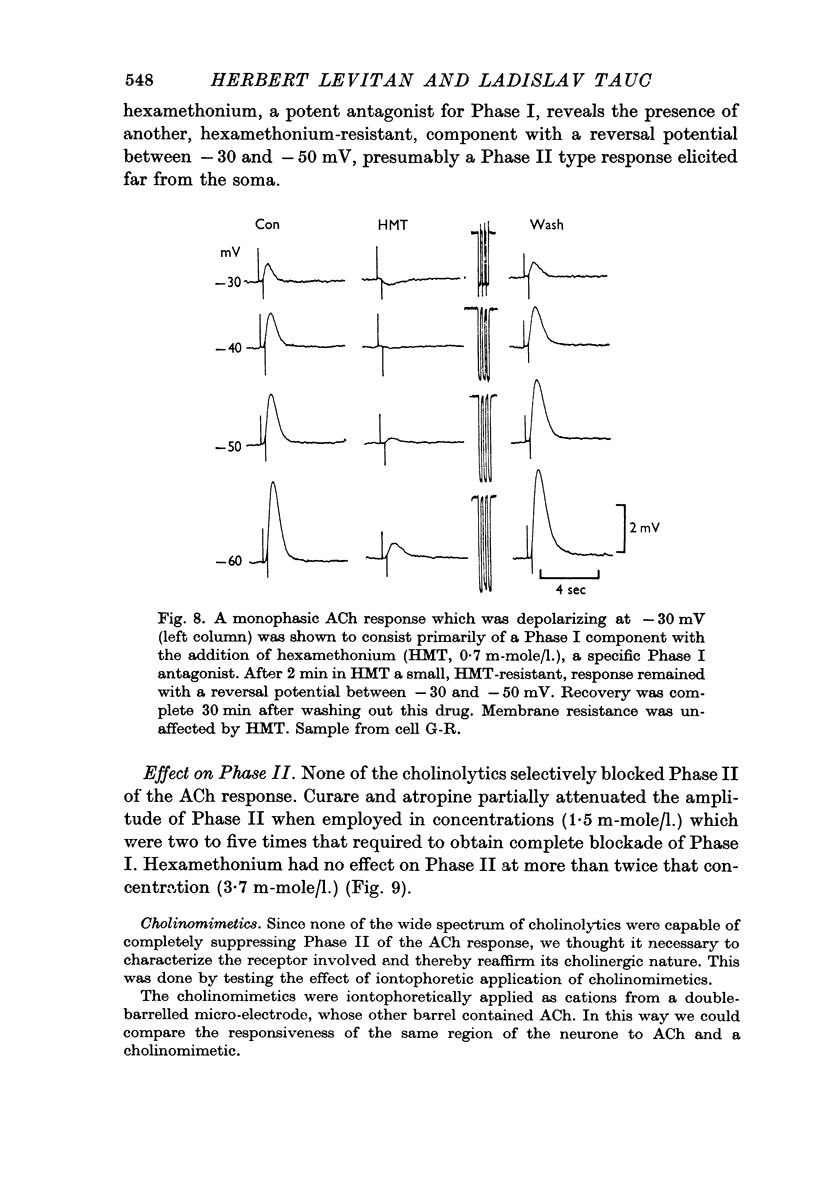
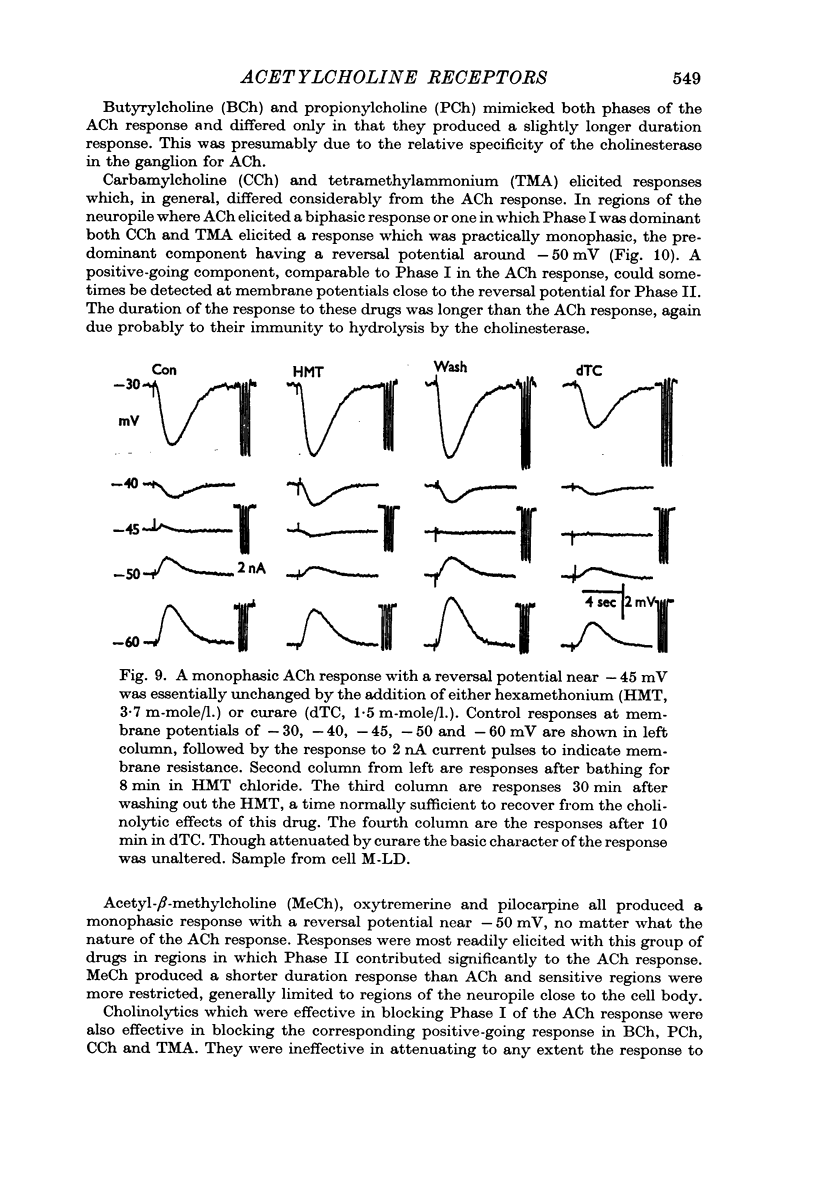
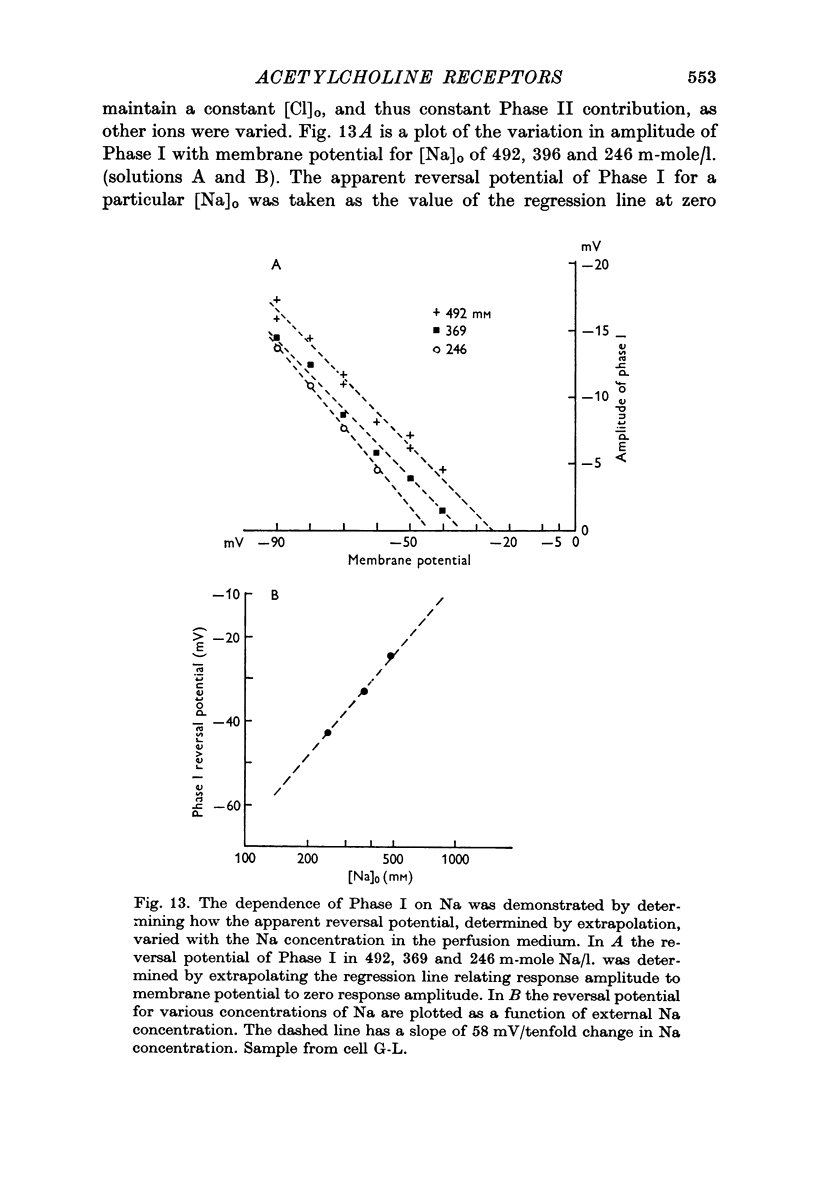
3. ACh applied more distal to the soma, in the neuropile, produced a 1-3 sec monophasic response whose reversal potential was more positive than - 30 mV, varied in amplitude with changes in the [Na]o of the medium, and was blocked by cholinolytics such as tubocurarine, hexamethonium and atropine.
4. With larger iontophoretic currents a biphasic response could be obtained, depolarization followed by hyperpolarization, which represented a superposition of the above monophasic potentials.
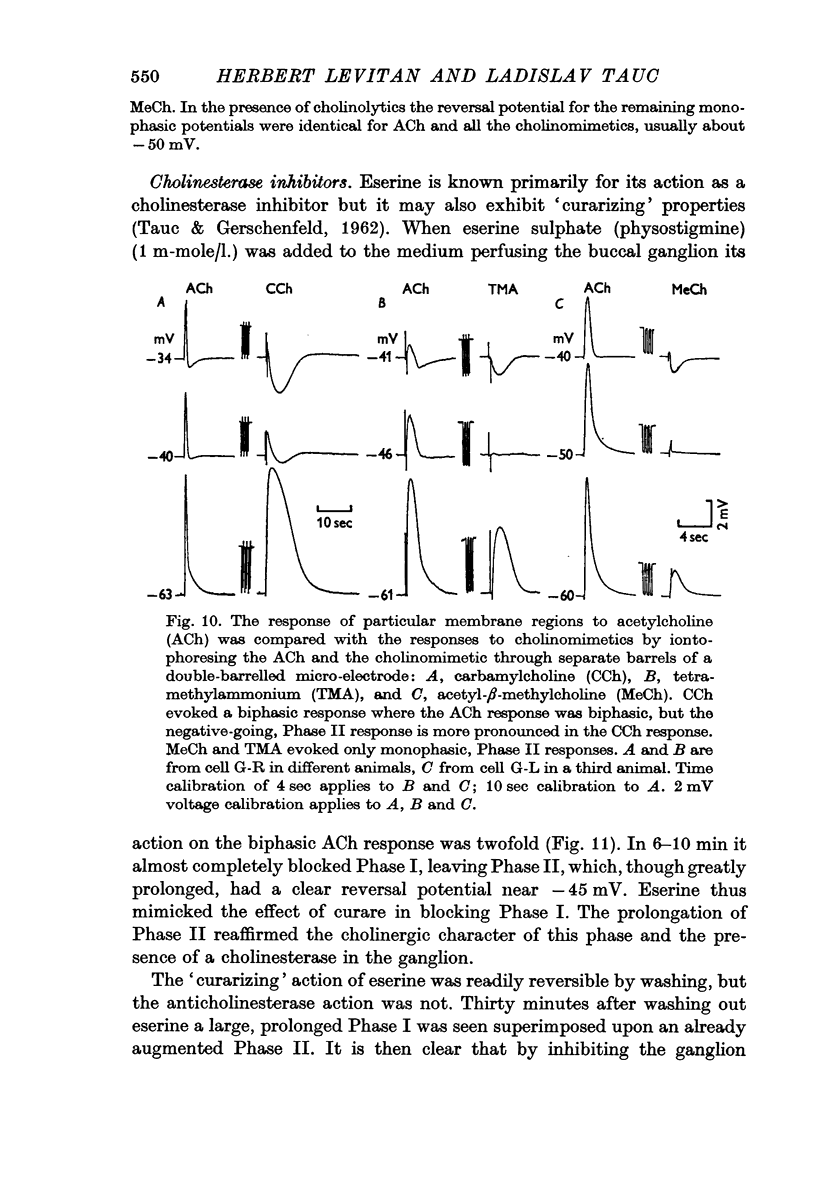
5. The cholinomimetics propionylcholine and butyrylcholine caused a biphasic response like that to ACh. Carbamylcholine and tetramethylammonium also produced a biphasic response but with a more prominent Cl component than that to ACh. Acetyl-β-methylcholine, oxytremorine and pilocarpine only produced a response comparable to the chloride phase of the ACh response.
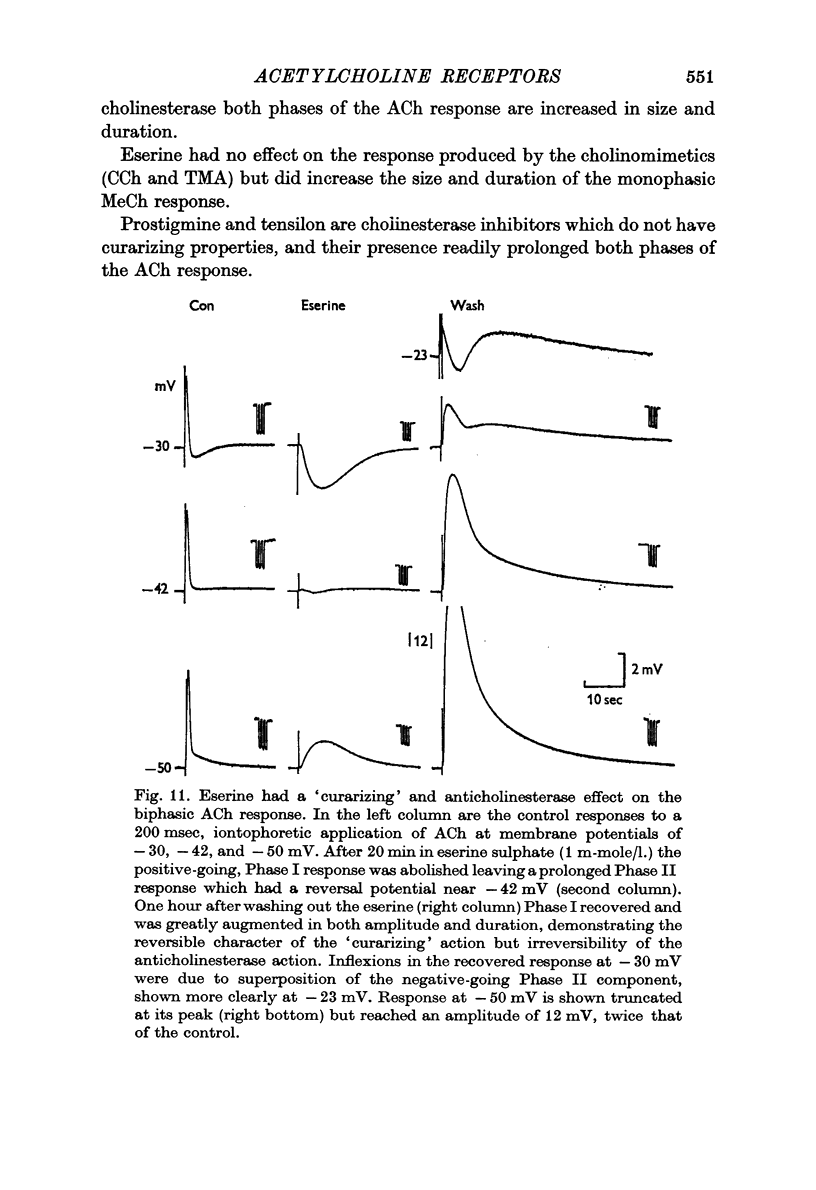
6. Anticholinesterases prolonged both phases of the ACh response.
7. It was concluded that each of the identified neurones possess two types of cholinoceptive sites, which are pharmacologically distinct, produced different changes in membrane permeability and are distributed differently over the axo-somatic membrane complex.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Crayton J. W., Nicoll R. A. Supraoptic neurosecretory cells: adrenergic and cholinergic sensitivity. Science. 1971 Jan 15;171(3967):208–210. doi: 10.1126/science.171.3967.208. [DOI] [PubMed] [Google Scholar]
- Blankenship J. E., Wachtel H., Kandel E. R. Ionic mechanisms of excitatory, inhibitory, and dual synaptic actions mediated by an identified interneuron in abdominal ganglion of Aplysia. J Neurophysiol. 1971 Jan;34(1):76–92. doi: 10.1152/jn.1971.34.1.76. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The acetylcholine receptors of Renshaw cells. Exp Brain Res. 1966;2(1):66–80. doi: 10.1007/BF00234361. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Effets inhibiteurs et biphasiques de la sérotonine sur les neurones d'escargot. J Physiol (Paris) 1970;62 (Suppl 1):156–156. [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Single presynaptic neurone mediates a two component postsynaptic inhibition. Nature. 1969 Mar 1;221(5183):866–868. doi: 10.1038/221866a0. [DOI] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Levitan H., Tauc L., Segundo J. P. Electrical transmission among neurons in the buccal ganglion of a mollusc, Navanax inermis. J Gen Physiol. 1970 Apr;55(4):484–496. doi: 10.1085/jgp.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmansohn D. Proteins in excitable membranes: their properties and function in bioelectricity are discussed. Science. 1970 May 29;168(3935):1059–1066. doi: 10.1126/science.168.3935.1059. [DOI] [PubMed] [Google Scholar]
- Stefani E., Gerschenfeld H. M. Comparative study of acetylcholine and 5-hydroxytryptamine receptors on single snail neurons. J Neurophysiol. 1969 Jan;32(1):64–74. doi: 10.1152/jn.1969.32.1.64. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TAUC L. [Maintenance of synaptic transmission in the giant neuron of Aplysia without activation of the soma or in the absence of the soma]. C R Hebd Seances Acad Sci. 1960 Feb 22;250:1560–1562. [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. Conversion of synaptic excitation to inhibition at a dual chemical synapse. J Neurophysiol. 1971 Jan;34(1):56–68. doi: 10.1152/jn.1971.34.1.56. [DOI] [PubMed] [Google Scholar]


