Abstract
1. The onset and development of transmission has been studied electro-physiologically in the isolated chick ciliary ganglion from Stage 25 (Hamburger & Hamilton, 1951) until 28 days after hatching. Ultrastructure of the synapses was concomitantly investigated.
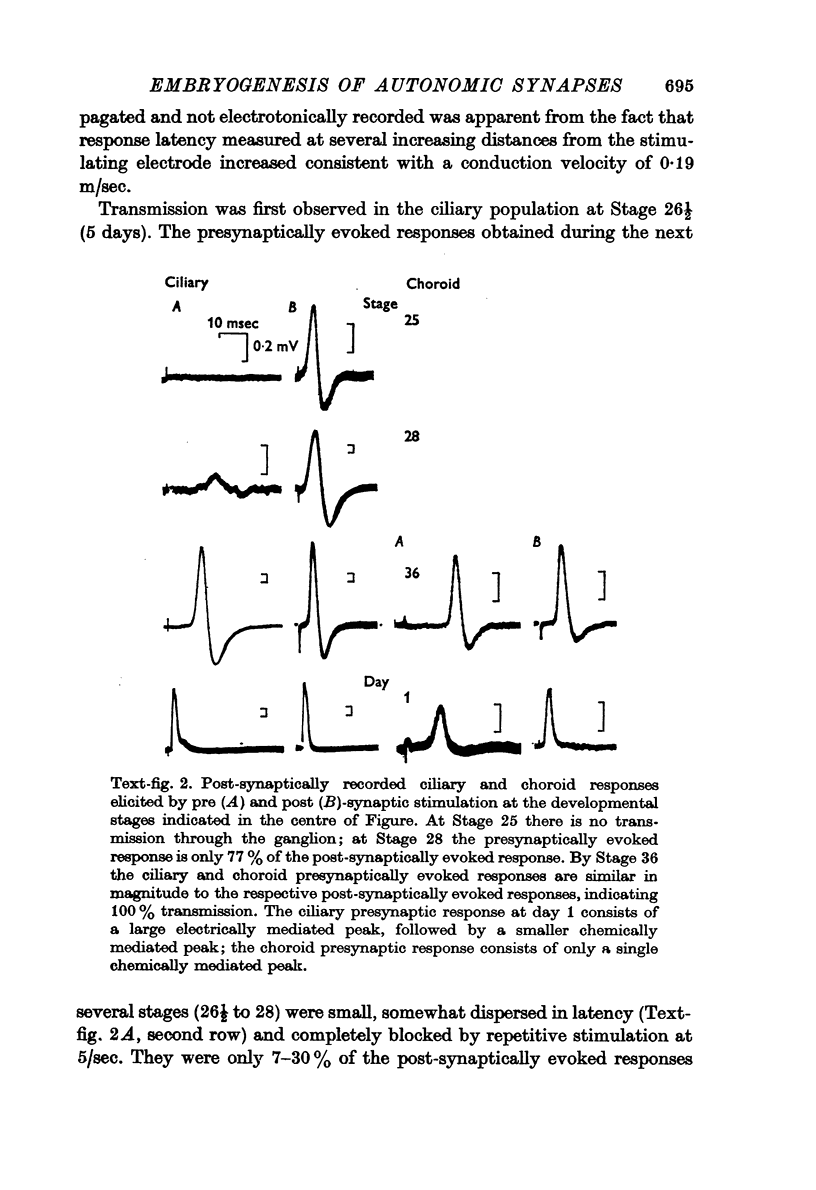
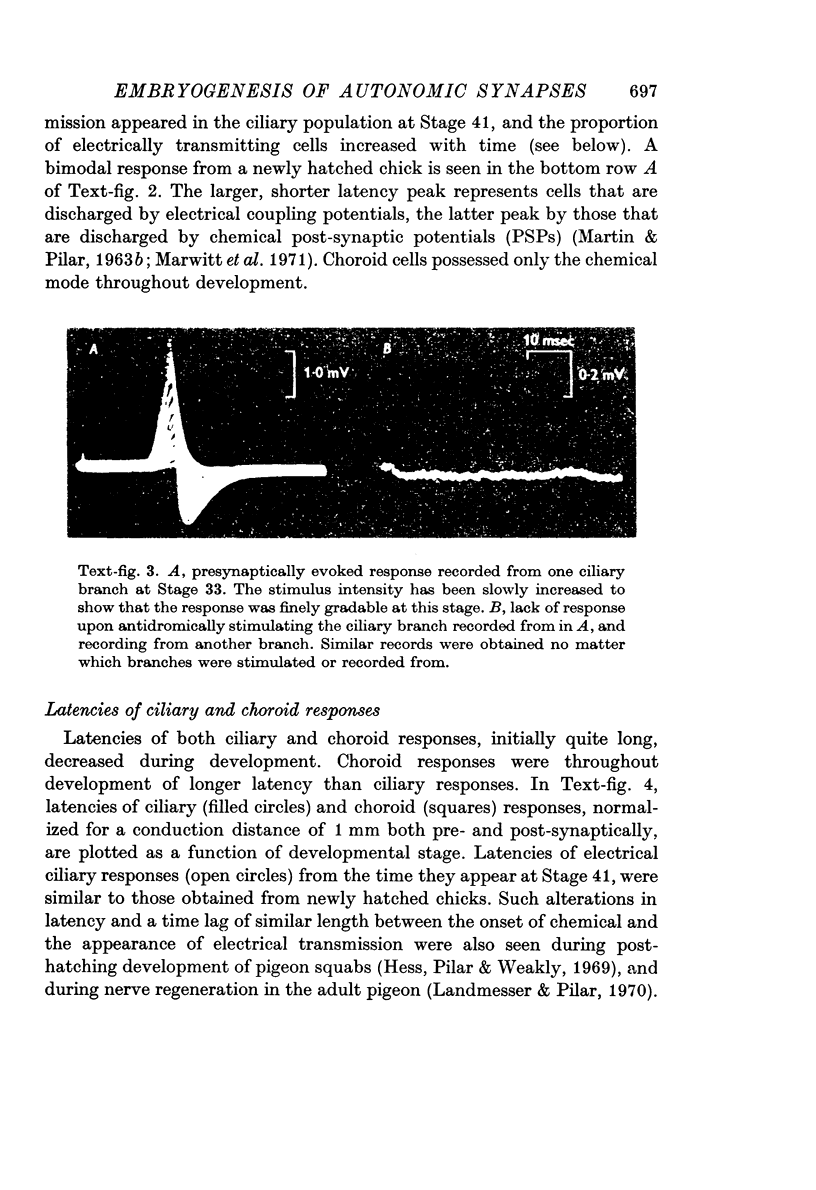
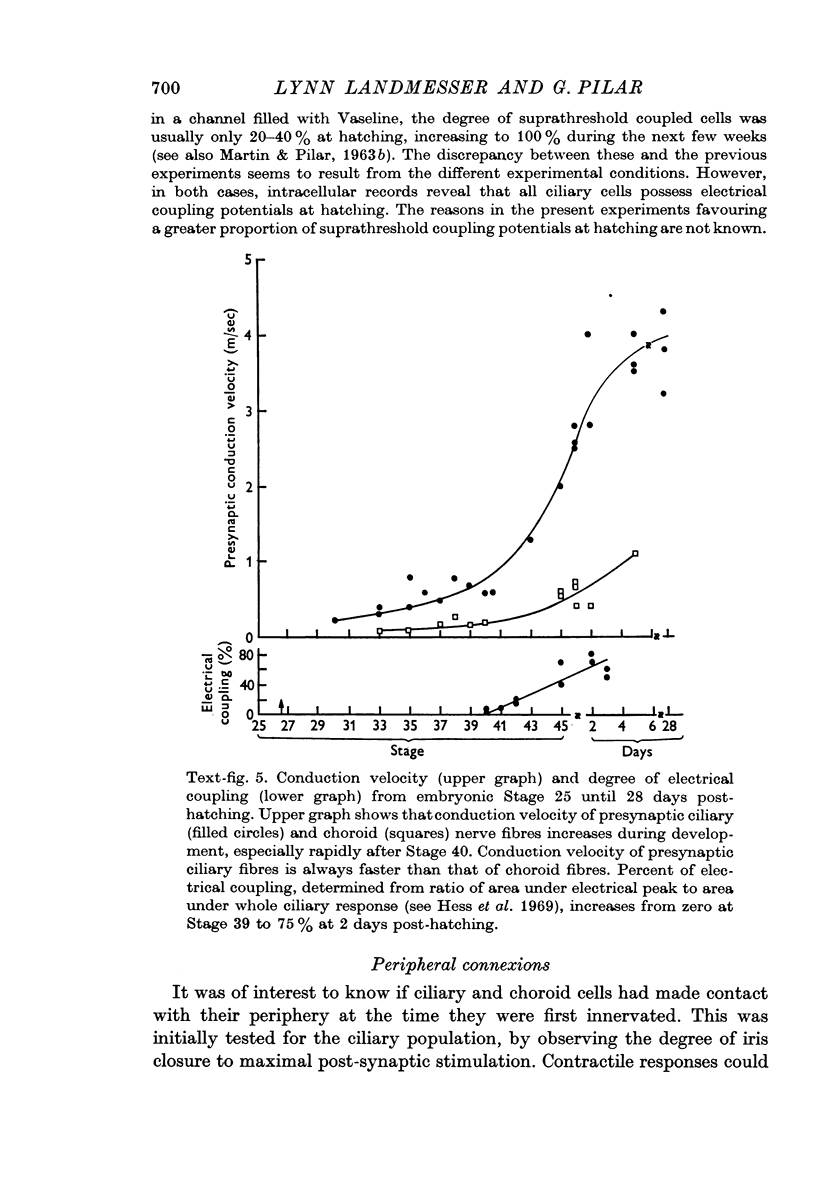
2. Synaptic transmission began at Stage 26½ and was 100% in both cell groups, ciliary and choroid, by Stage 33. It was initially chemical until Stage 41 when effective electrical coupling first appeared in the ciliary population. The proportion of electrically transmitting synapses increased to 80% by 1-2 days post-hatching.
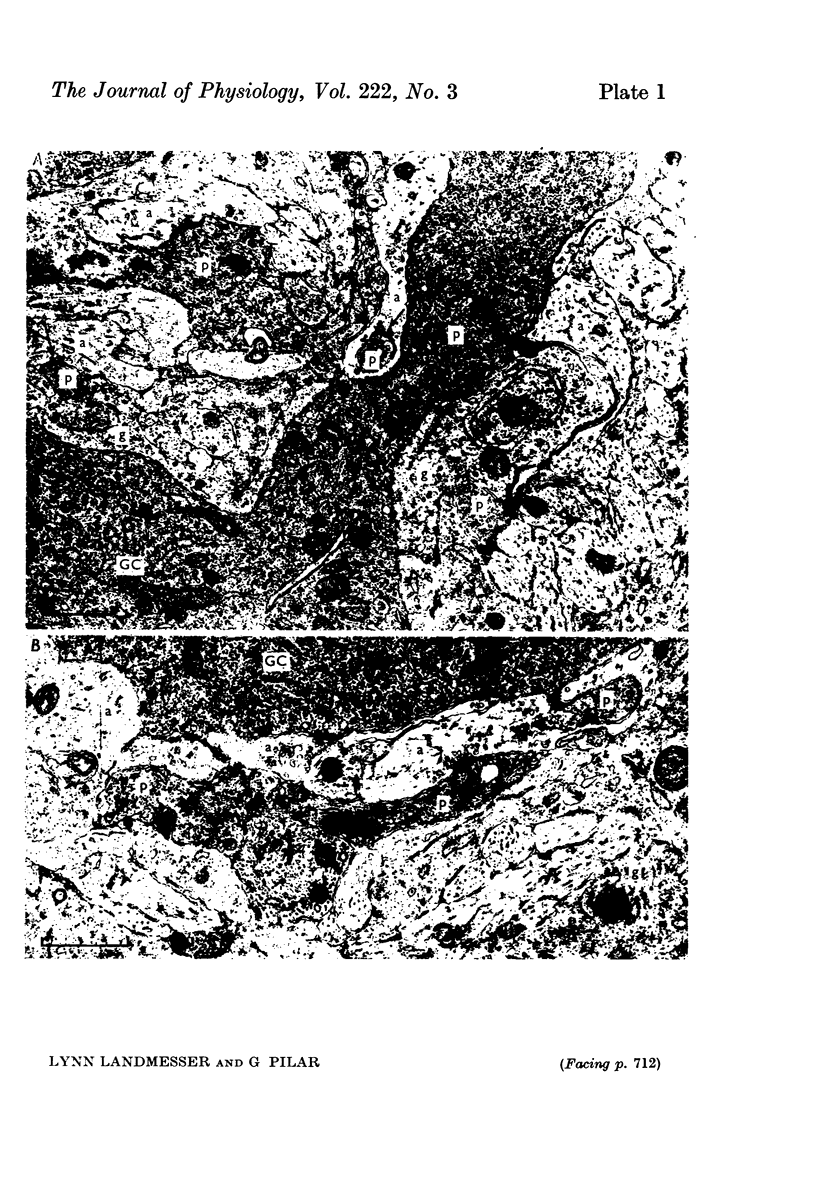
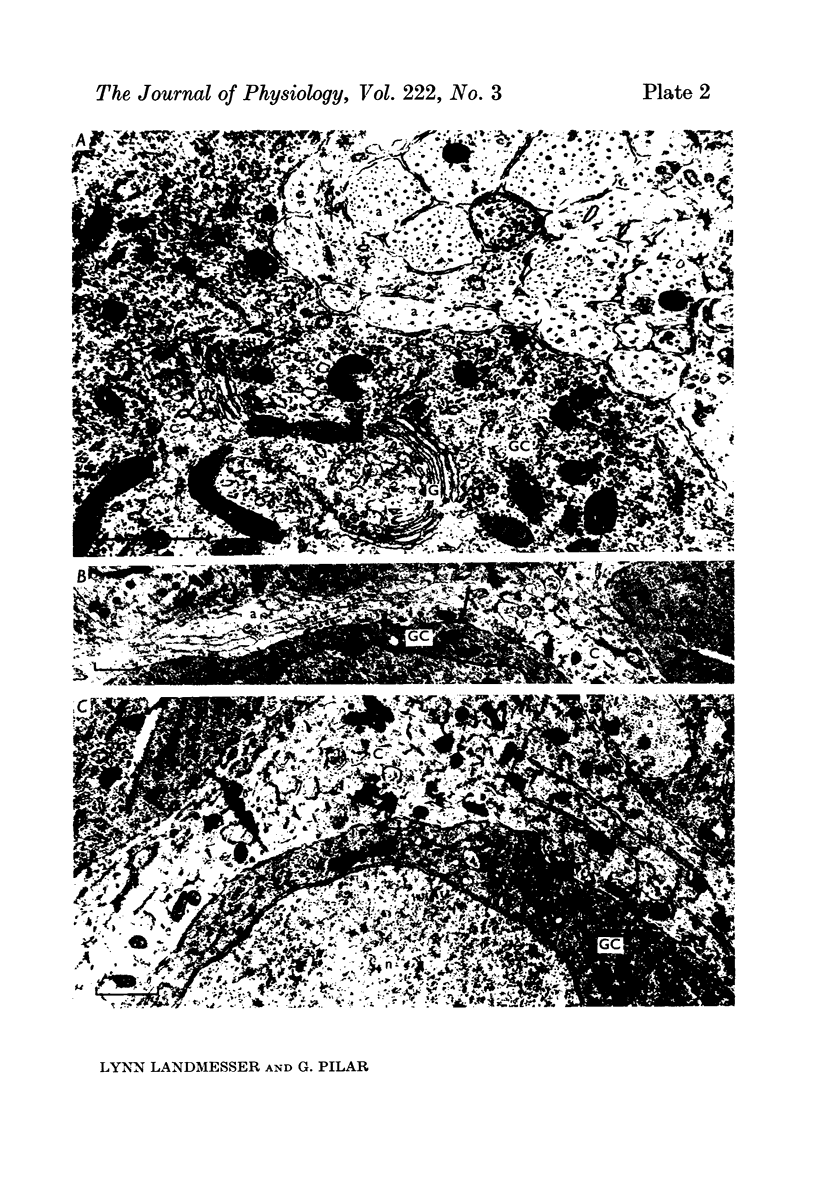
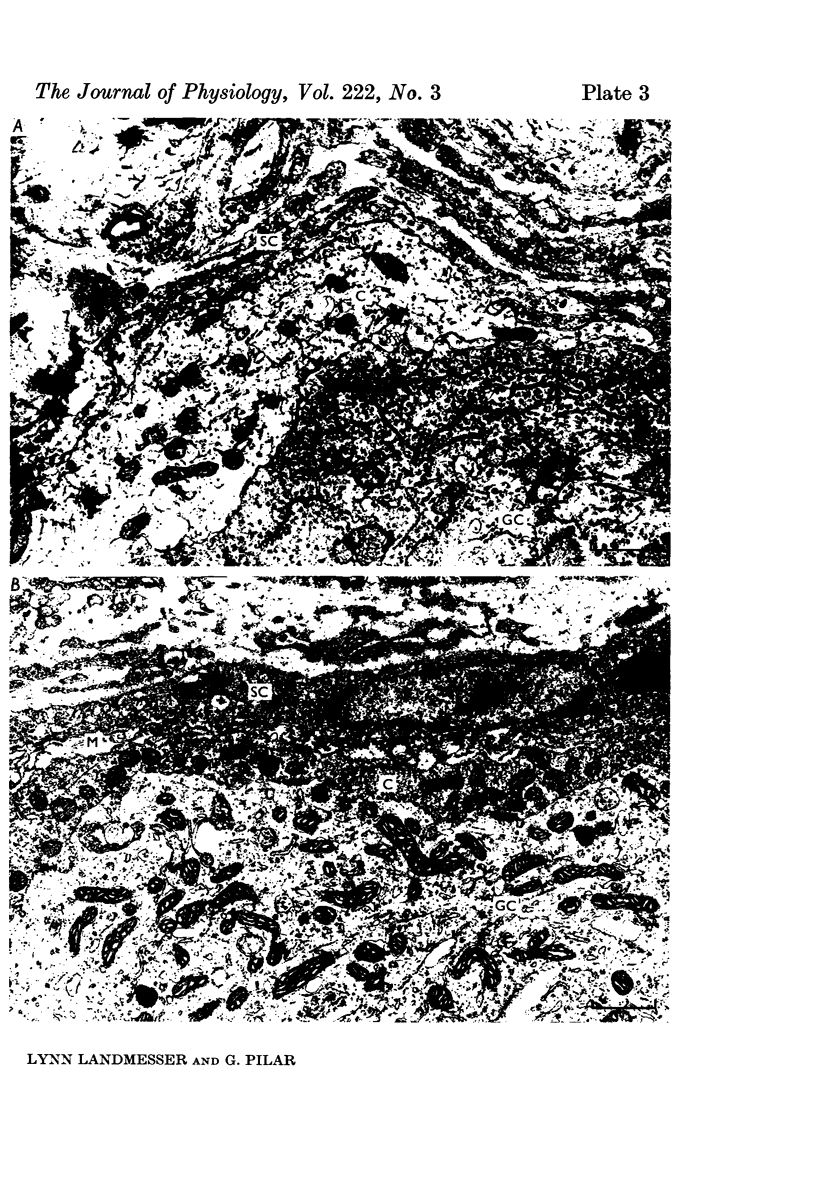
3. Few morphological synapses were present at Stage 33½ when all ganglion cells were transmitting. A scarcity of synaptic vesicles persisted until late in embryonic development when all ciliary cells possessed calyces. At hatching the calyces were filled with synaptic vesicles.
4. Initial synaptic contacts were by fine terminal branches often on the intricate processes of early ganglion cells. Calyces formed from Stage 36½ and there was a concomitant retraction of ganglion cell processes, so that by Stage 40 all ciliary cells had simple calyces. The calyx was a transitory structure, which from the first week post-hatching began to break up into a cluster of boutons.
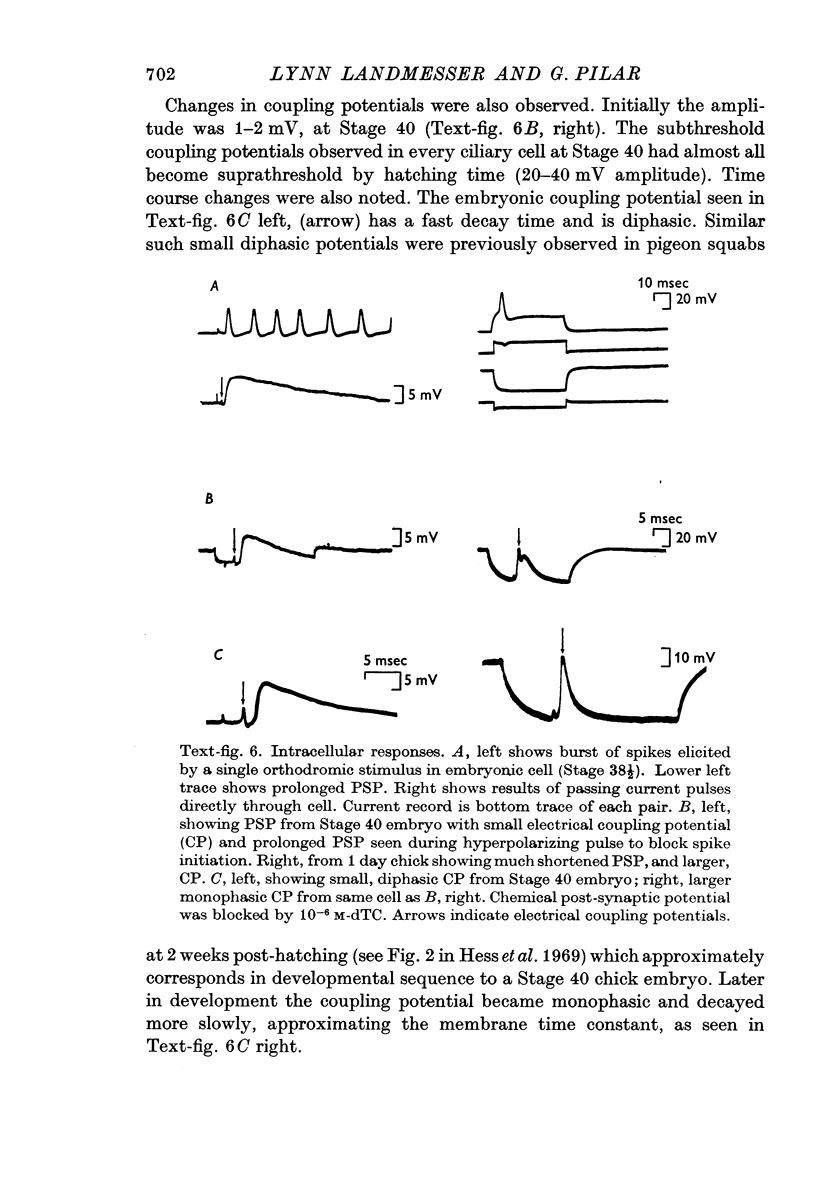
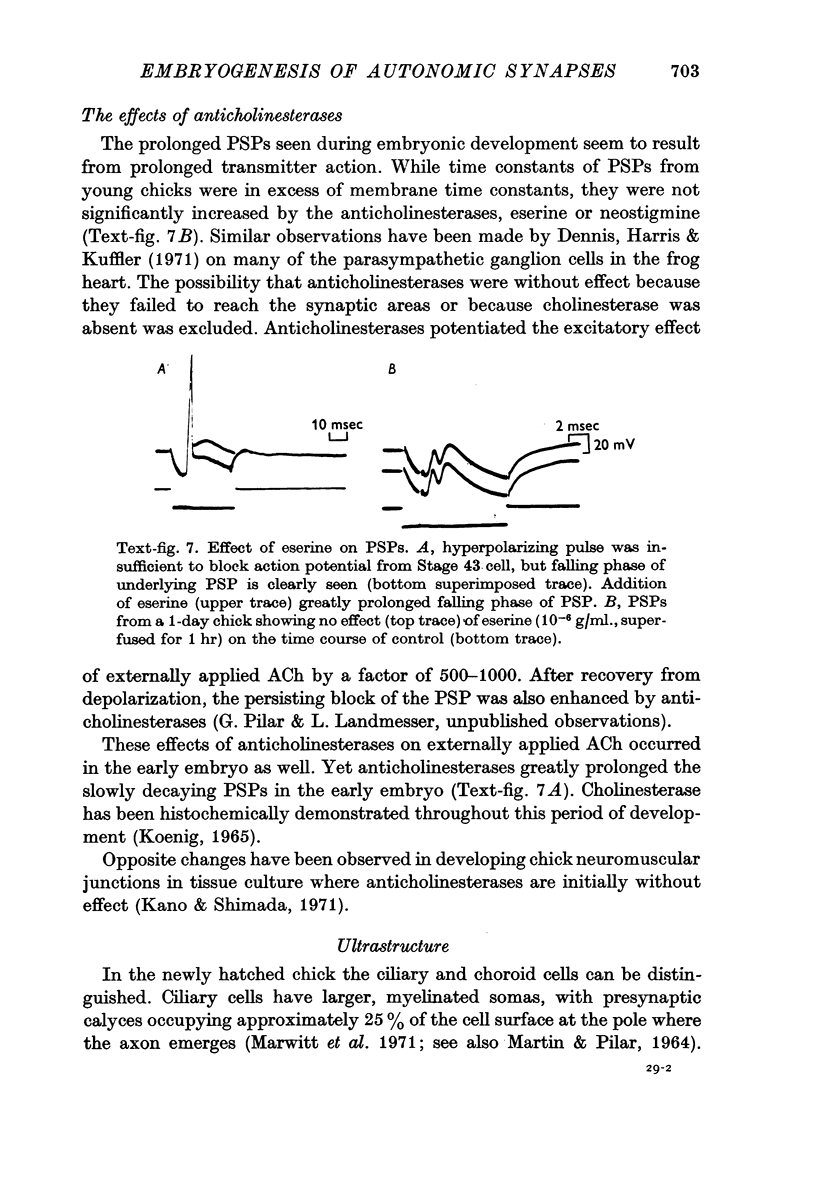
5. Chemical post-synaptic potentials (PSPs) were at Stage 40 long (30 × the membrane time constant) and further prolonged by eserine. By Stage 43, PSPs had become markedly shortened and were unaffected by eserine. No simple explanation can be offered for the changes in PSP time course and sensitivity to anticholinesterases during development.
6. Intracellular records from Stage 40 ciliary cells, which all possess calyces, showed 1-2 mV amplitude, diphasic, fast decaying electrical coupling potentials (CPs). Later in development the CPs became 20-40 mV amplitude, more slowly decaying and monophasic. This seemed to be correlated with faster presynaptic conduction velocities and myelination of the cell soma. Such changes in CPs may reflect a shift from capacitative to more resistive coupling and point to several factors contributing in varying degrees to the electrical transmission.
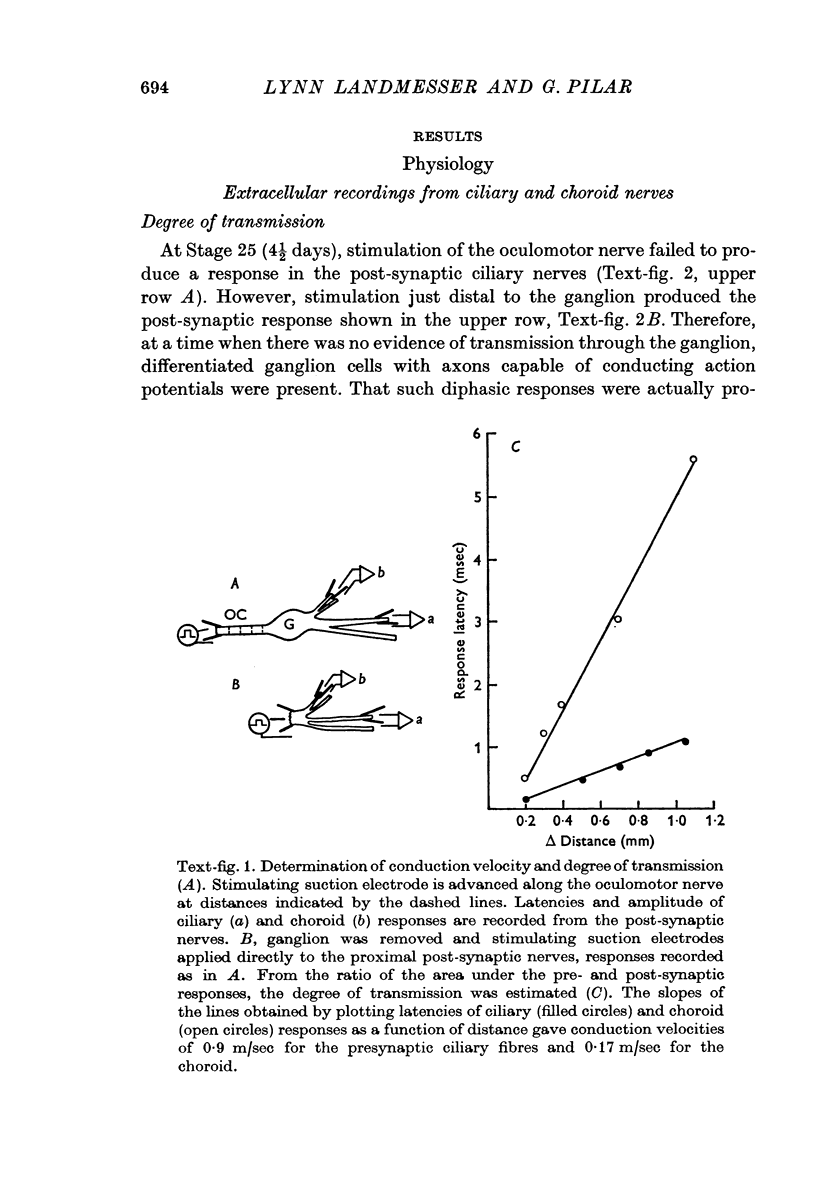
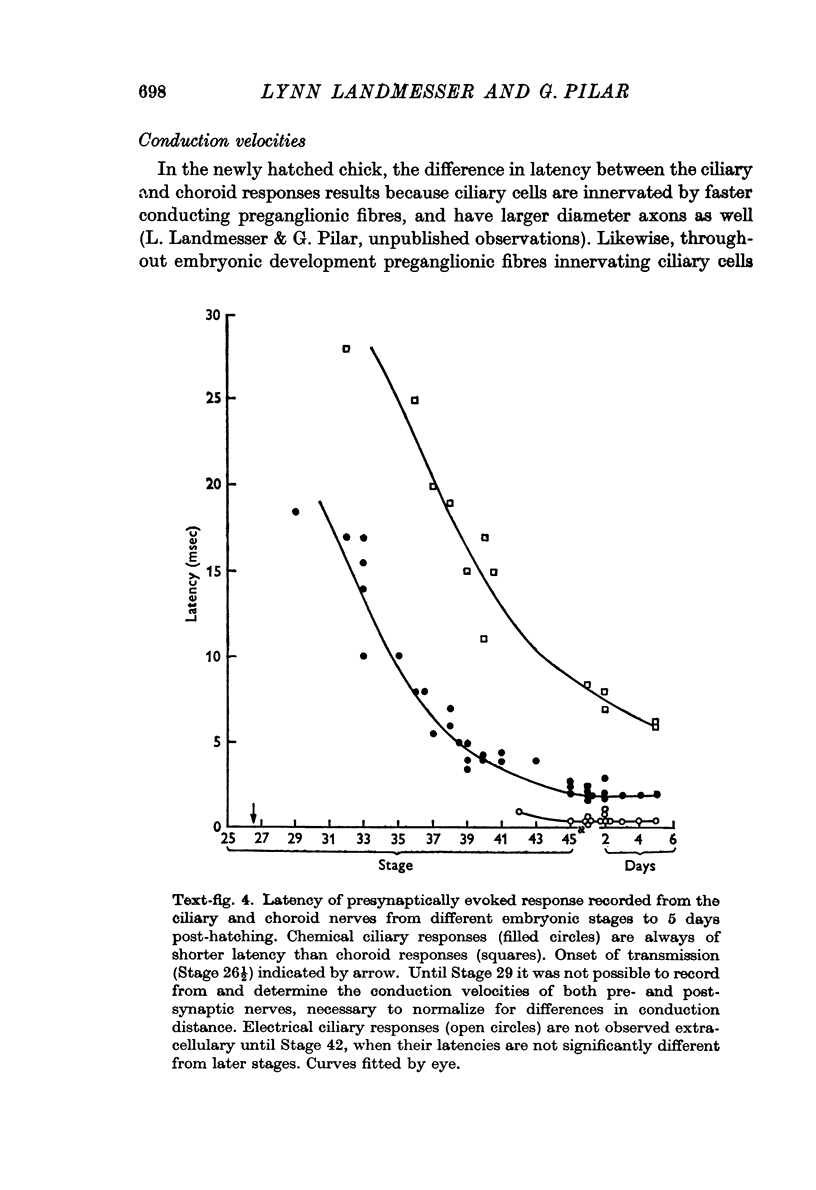
7. Presynaptic fibres innervating ciliary cells were from the start of lower threshold and faster conduction velocity than those innervating ciliary cells, as occurred in the adult. It is concluded that these preganglionic fibres were probably specified by the time transmission starts and that they selectively innervated the proper post-synaptic cells.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodian D. Development of fine structure of spinal cord in monkey fetuses. II. Pre-reflex period to period of long intersegmental reflexes. J Comp Neurol. 1968 Jun;133(2):113–166. doi: 10.1002/cne.901330202. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R. The onset of synapse formation in spinal cord cultures as studied by electron microscopy. Brain Res. 1967 Dec;6(4):728–749. doi: 10.1016/0006-8993(67)90129-1. [DOI] [PubMed] [Google Scholar]
- CARPENTER F. G., BERGLAND R. M. Excitation and conduction in immature nerve fibers of the developing chick. Am J Physiol. 1957 Aug;190(2):371–376. doi: 10.1152/ajplegacy.1957.190.2.371. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Wenger E. Degeneration in the nucleus of origin of the preganglionic fibers to the chick ciliary ganglion following early removal of the optic vesicle. J Exp Zool. 1968 May;168(1):105–123. doi: 10.1002/jez.1401680109. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Peterson E. R. Onset and development of functional interneuronal connections in explants of rat spinal cord-ganglia during maturation in culture. Brain Res. 1967 Dec;6(4):750–762. doi: 10.1016/0006-8993(67)90130-8. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Furness J. B., McLean J. R., Burnstock G. Distribution of adrenergic nerves and changes in neuromuscular transmission in the mouse vas deferens during postnatal development. Dev Biol. 1970 Apr;21(4):491–505. doi: 10.1016/0012-1606(70)90074-6. [DOI] [PubMed] [Google Scholar]
- GLEES P., SHEPPARD B. L. ELECTRON MICROSCOPICAL STUDIES OF THE SYNAPSE IN THE DEVELOPING CHICK SPINAL CORD. Z Zellforsch Mikrosk Anat. 1964 Apr 9;62:356–362. doi: 10.1007/BF00339285. [DOI] [PubMed] [Google Scholar]
- Hess A. Developmental changes in the structure of the synapse on the myelinated cell bodies of the chicken ciliary ganglion. J Cell Biol. 1965 Jun;25(3 Suppl):1–19. doi: 10.1083/jcb.25.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A., Pilar G., Weakly J. N. Correlation between transmission and structure in avian ciliary ganglion synapses. J Physiol. 1969 Jun;202(2):339–354. doi: 10.1113/jphysiol.1969.sp008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Shimada Y. Innervation of skeletal muscle cells differentiated in vitro from chick embryo. Brain Res. 1971 Apr 2;27(2):402–405. doi: 10.1016/0006-8993(71)90271-x. [DOI] [PubMed] [Google Scholar]
- Koenig H. L. Relations entre la distribution de l'activité acétylcholinestérasique et celle de l'ergastoplasme dans les neurones du ganglion ciliaire du poulet. Arch Anat Microsc Morphol Exp. 1965 Oct-Dec;54(4):937–963. [PubMed] [Google Scholar]
- Kuba K., Tomita T. Effect of prostigmine on the time course of the end-plate potential in the rat diaphragm. J Physiol. 1971 Mar;213(3):533–544. doi: 10.1113/jphysiol.1971.sp009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., AMPRINO R. Recherches expérimentales sur l'origine du ganglion ciliaire dans l'embryon de poulet. Arch Biol (Liege) 1947;58(3):265–288. [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Selective reinnervation of two cell populations in the adult pigeon ciliary ganglion. J Physiol. 1970 Nov;211(1):203–216. doi: 10.1113/jphysiol.1970.sp009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L. Fine structure of nerves in the regenerating limb of the newt Triturus. Am J Anat. 1967 Nov;121(3):647–669. doi: 10.1002/aja.1001210312. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. AN ANALYSIS OF ELECTRICAL COUPLING AT SYNAPSES IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Jun;171:454–475. doi: 10.1113/jphysiol.1964.sp007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. TRANSMISSION THROUGH THE CILIARY GANGLION OF THE CHICK. J Physiol. 1963 Sep;168:464–475. doi: 10.1113/jphysiol.1963.sp007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Morest D. K. The growth of synaptic endings in the mammalian brain: a study of the calyces of the trapezoid body. Z Anat Entwicklungsgesch. 1968 Nov 4;127(3):201–220. doi: 10.1007/BF00526129. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. Developmental aspects of synaptology with special emphasis upon the cerebellar cortex. UCLA Forum Med Sci. 1971;14:141–165. [PubMed] [Google Scholar]
- Pannese E. Temporary junctions between neoroblasts in the developing spinal ganglia of the domestic fowl. J Ultrastruct Res. 1967 Dec 12;21(3):233–250. doi: 10.1016/s0022-5320(67)80094-7. [DOI] [PubMed] [Google Scholar]
- Pilar G., Vaughan P. C. Ultrastructure and contractures of the pigeon iris striated muscle. J Physiol. 1971 Dec;219(2):253–266. doi: 10.1113/jphysiol.1971.sp009660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Yonezawa T. Physiological studies during formation and development of rat neuromuscular junctions in tissue culture. J Gen Physiol. 1971 Oct;58(4):467–481. doi: 10.1085/jgp.58.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. D. Electrophysiological evidence for low-resistance intercellular junctions in the early chick embryo. J Cell Biol. 1968 Jun;37(3):650–659. doi: 10.1083/jcb.37.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Hama K. Some observations on the fine structure of the synaptic area in the ciliary ganglion of the chick. Z Zellforsch Mikrosk Anat. 1965 Jul 15;67(2):174–184. doi: 10.1007/BF00344467. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol. 1970 Jan;44(1):62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler W., Schmekel L. Elektronenmikroskopische Untersuchung der Entwicklung der vegetativen (Grenzstrang-) und spinalen Ganglien bei Gallus domesticus. Acta Neuroveg (Wien) 1967;30(1):427–444. doi: 10.1007/BF01239924. [DOI] [PubMed] [Google Scholar]