Abstract
1. The entoptic shadows of the retinal blood vessels were visualized by temporal modulation of their contrast.
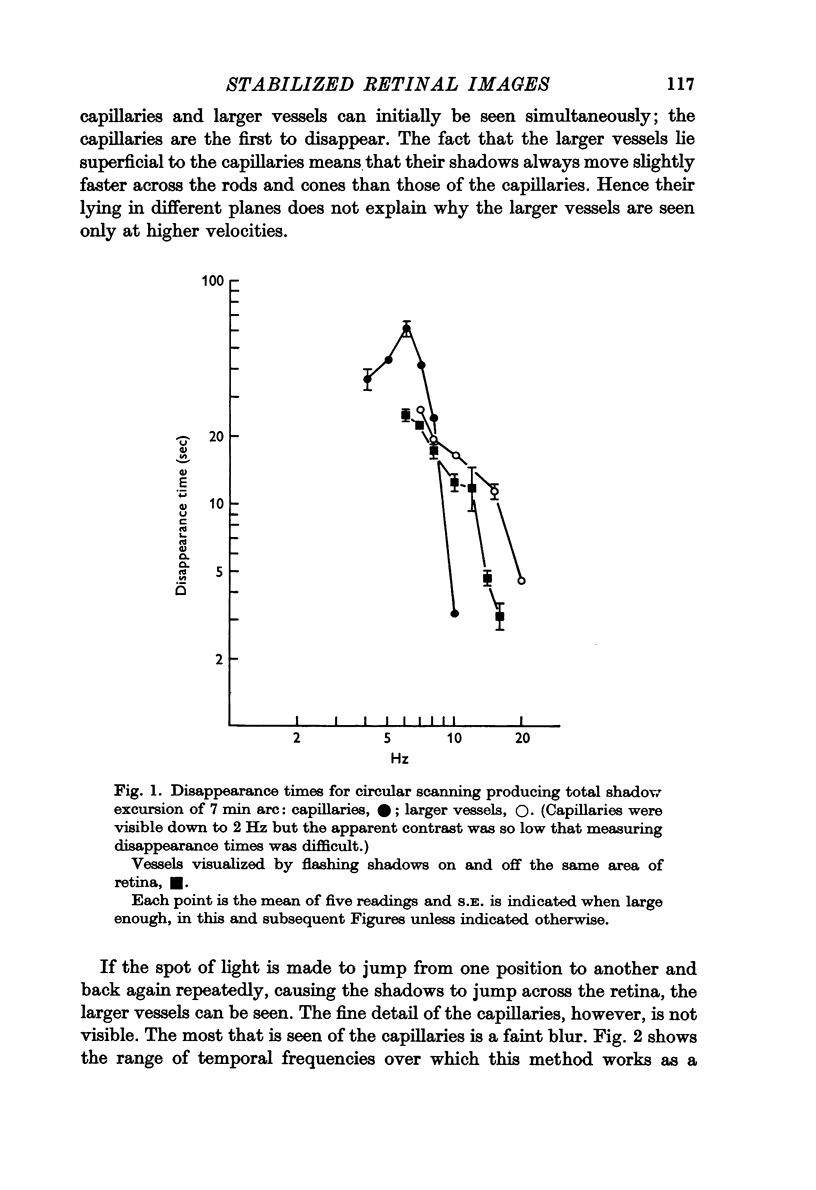
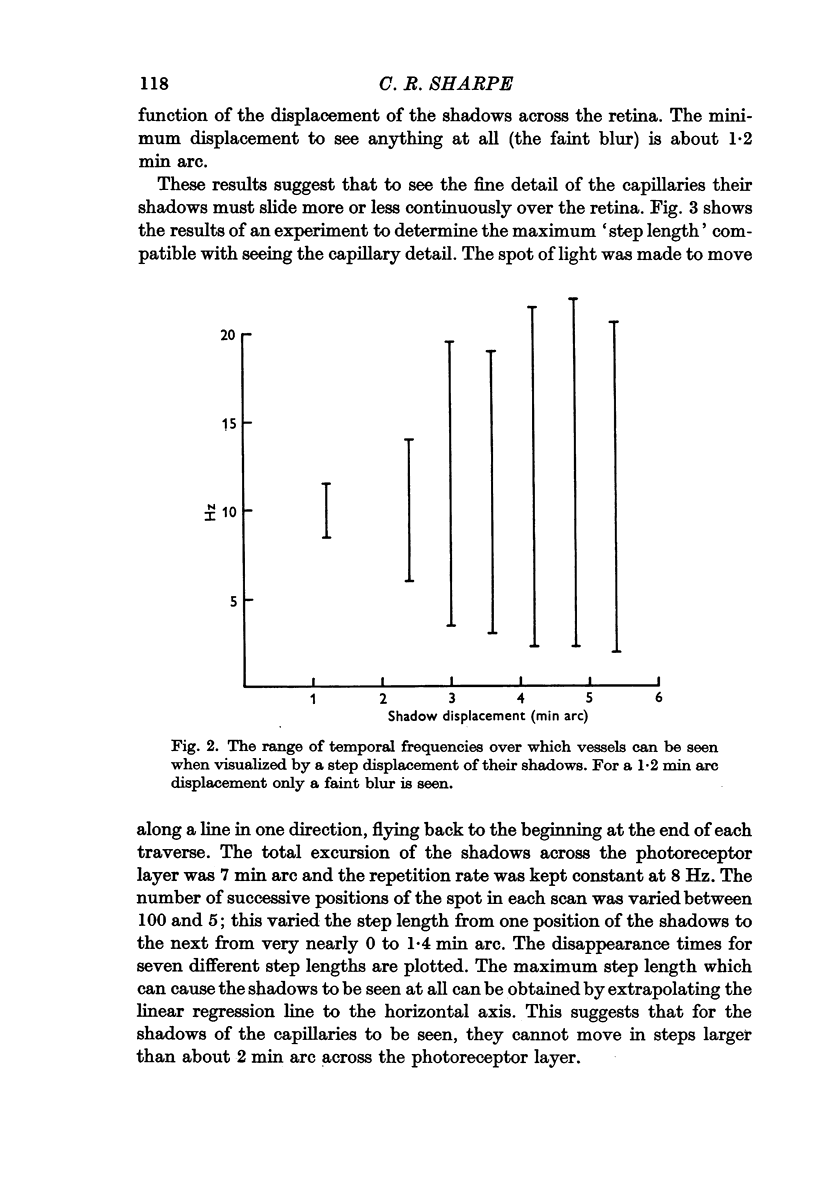
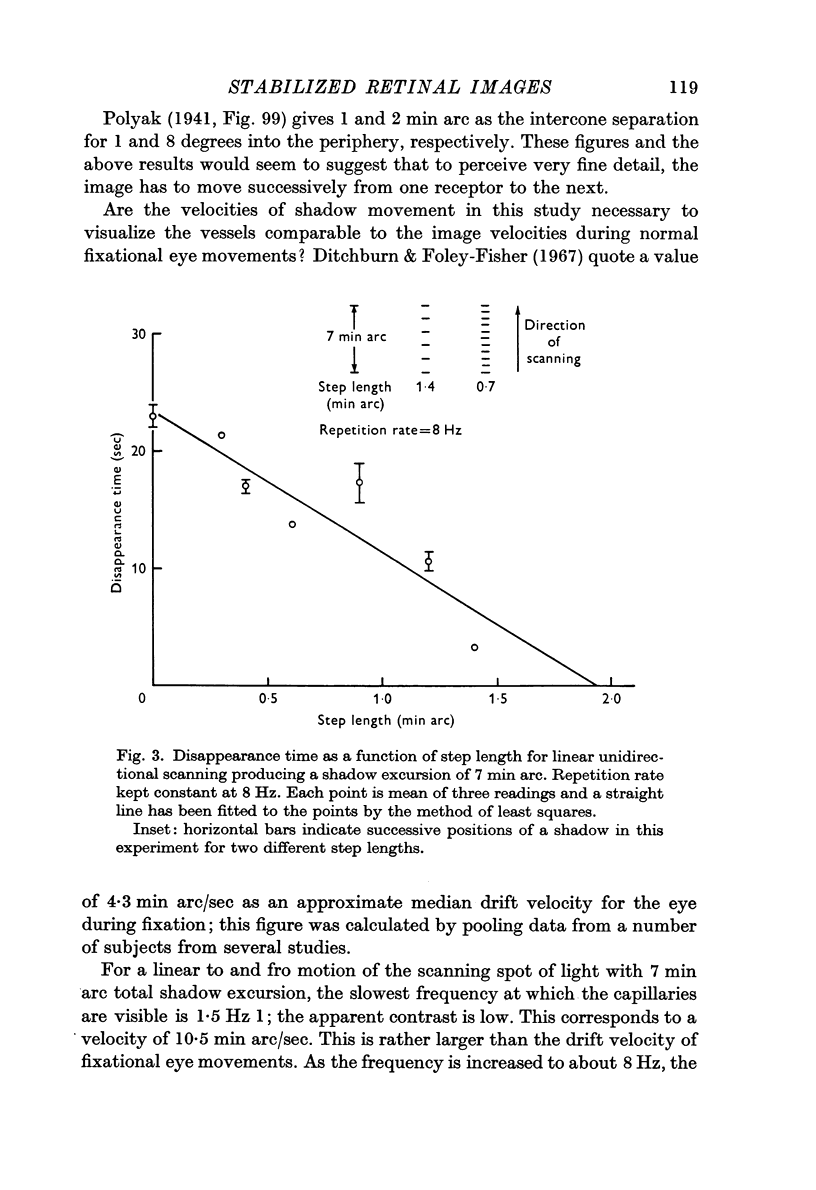
2. For perception of fine detail, the shadows must move successively from one photoreceptor to the next. To see coarser detail the contrast need only be temporally modulated.
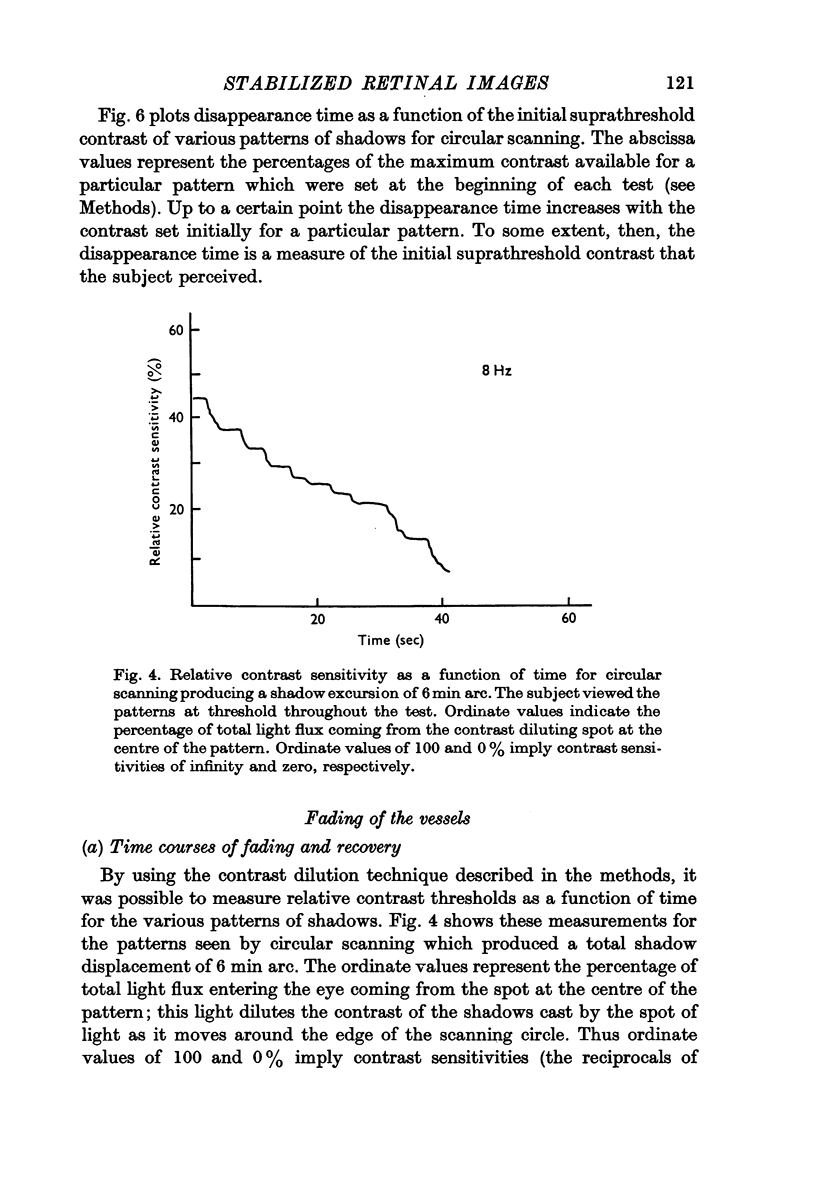
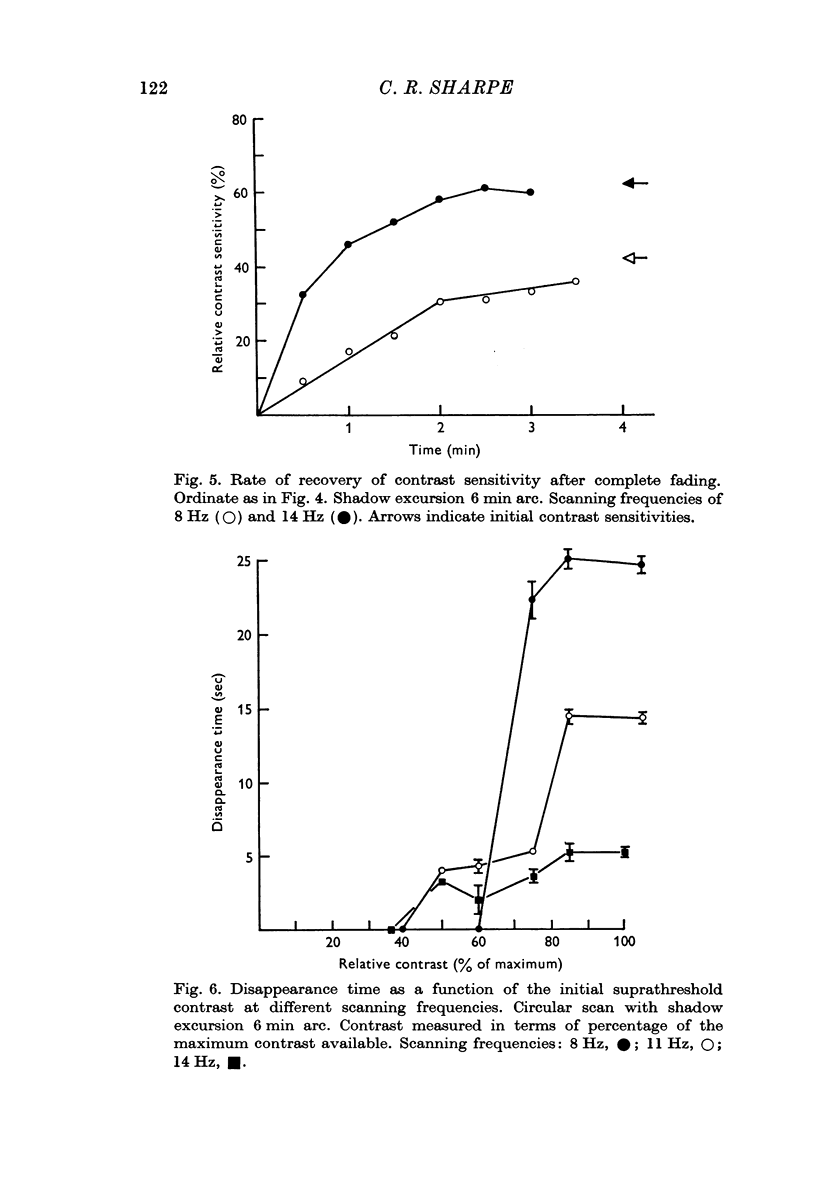
3. The contrast threshold of the pattern of shadows rises steadily as they are viewed until the shadows can no longer be seen even with the highest contrast available.
4. This elevation of contrast threshold is partially binocularly transferred, suggesting that the perceptual fading has a central origin.
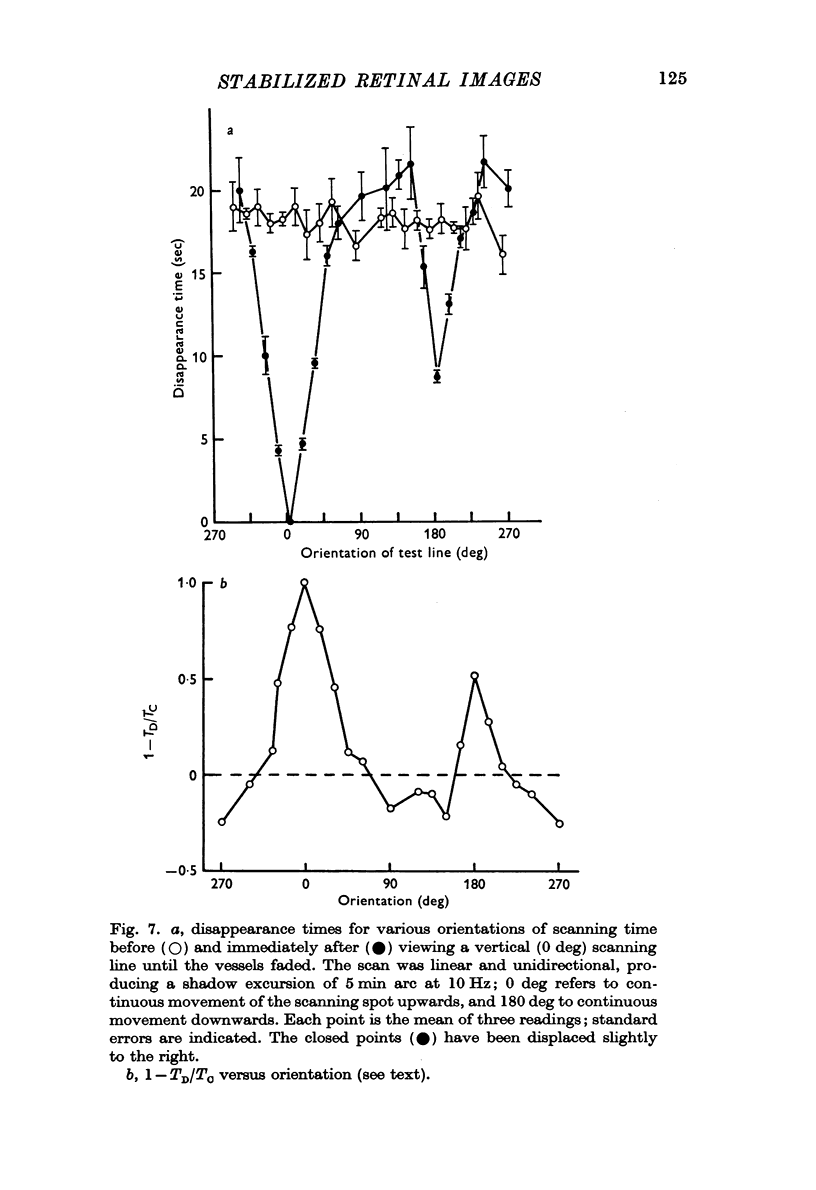
5. The fading of the shadows is specific for their orientation, direction of movement and their width.
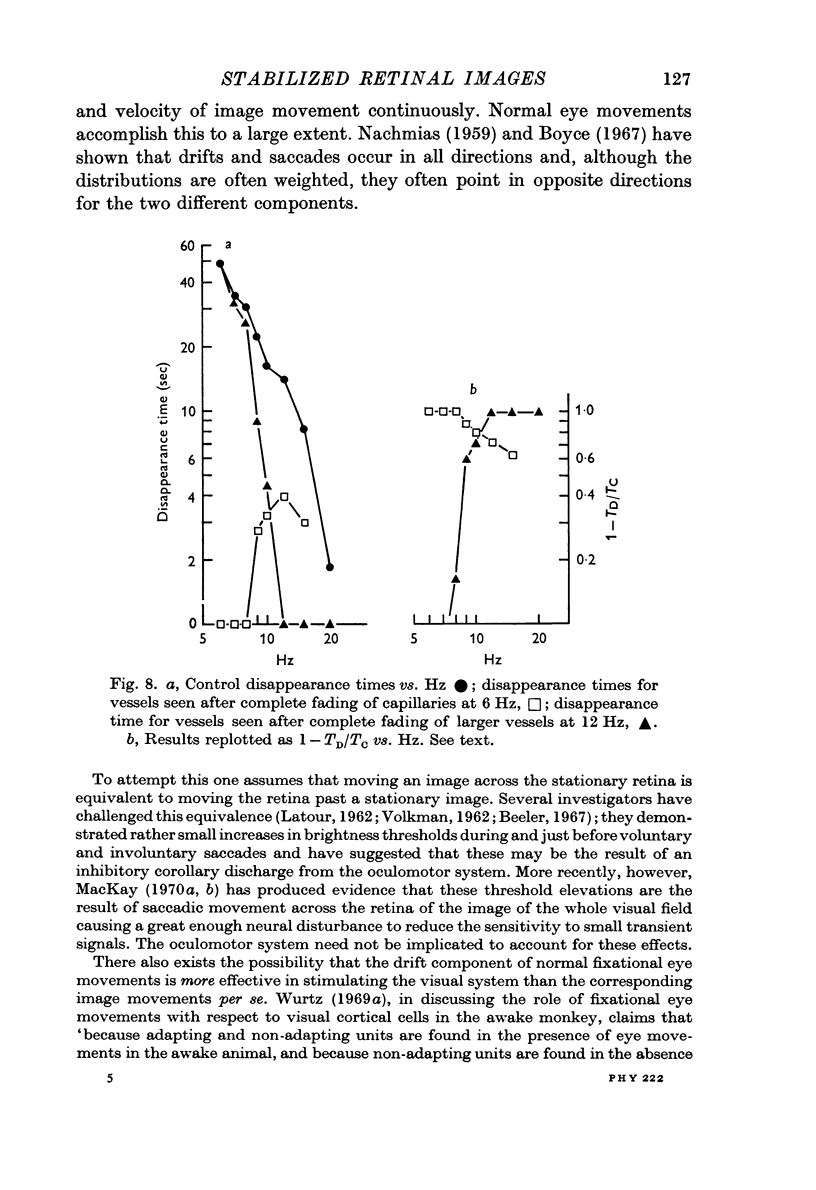
6. Image movements like those produced by fixational eye movements have been simulated. The shadows still fade; the results can be explained in terms of spatial adaptation (Blakemore & Campbell, 1969) of spatial frequency and orientation channels.
Full text
PDF






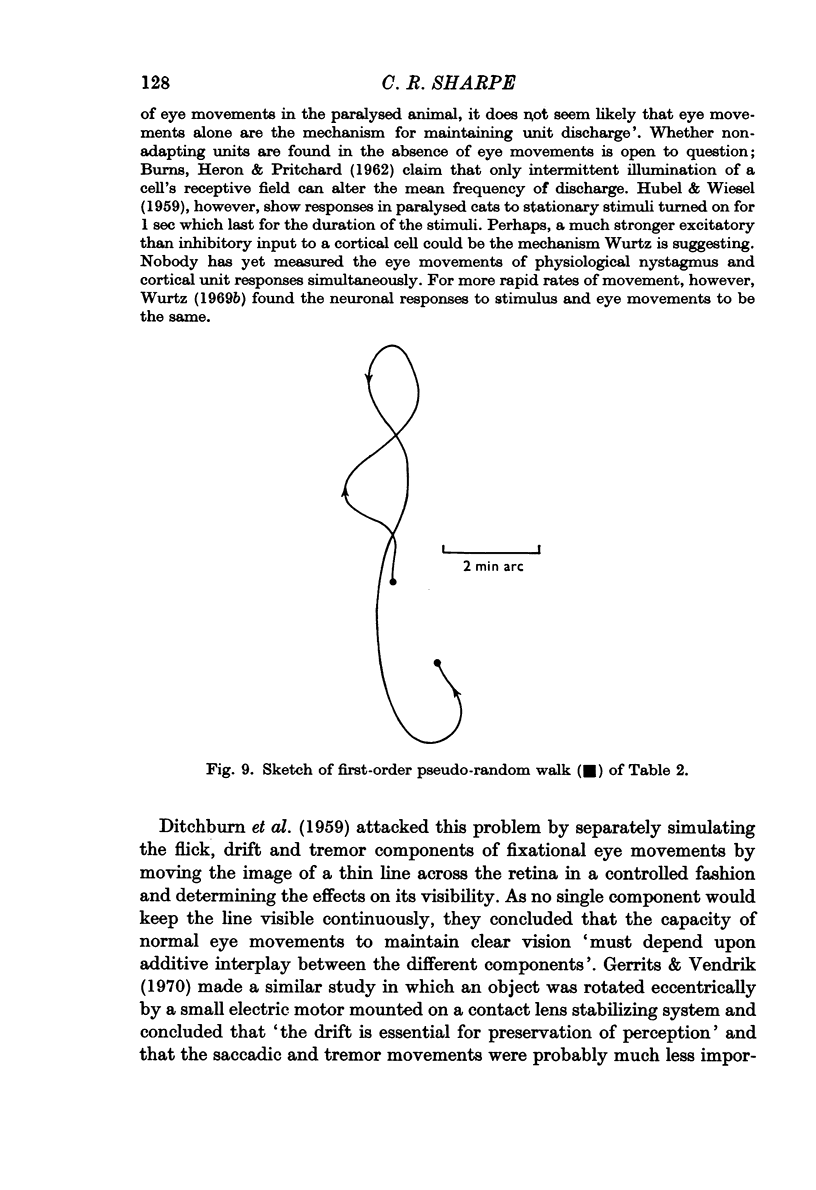

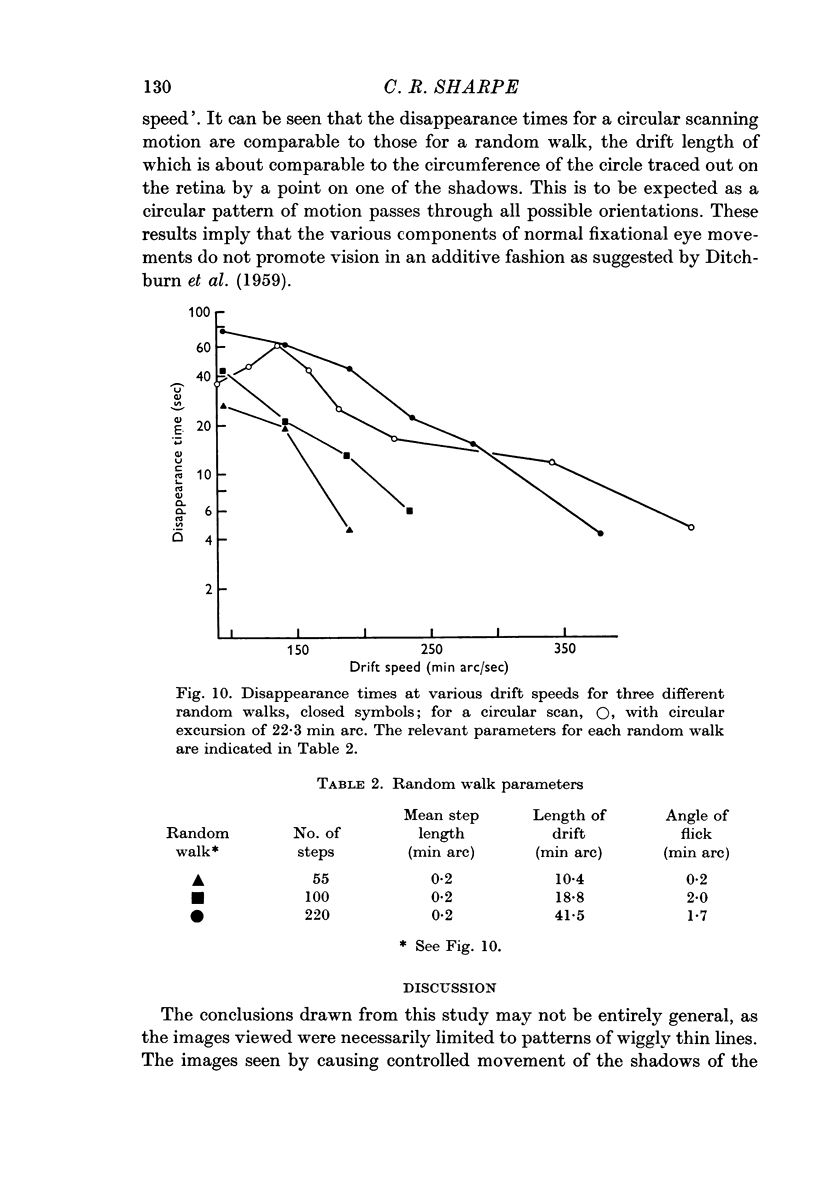




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., BRINDLEY G. S. INTER-OCULAR TRANSFER OF MOVEMENT AFTER-EFFECTS DURING PRESSURE BLINDING OF THE STIMULATED EYE. Nature. 1963 Dec 28;200:1347–1347. doi: 10.1038/2001347a0. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M. EVIDENCE FOR A PHYSIOLOGICAL EXPLANATION OF THE WATERFALL PHENOMENON AND FIGURAL AFTER-EFFECTS. Nature. 1963 Dec 28;200:1345–1347. doi: 10.1038/2001345a0. [DOI] [PubMed] [Google Scholar]
- BEHRENDT T., WILSON L. A. SPECTRAL REFLECTANCE PHOTOGRAPHY OF THE RETINA. Am J Ophthalmol. 1965 Jun;59:1079–1088. [PubMed] [Google Scholar]
- BURNS B. D., HERON W., PRITCHARD R. Physiological excitation of visual cortex in cat's unanesthetized isolated forebrain. J Neurophysiol. 1962 Mar;25:165–181. doi: 10.1152/jn.1962.25.2.165. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr Visual threshold changes resulting from spontaneous saccadic eye movements. Vision Res. 1967 Sep;7(9):769–775. doi: 10.1016/0042-6989(67)90039-9. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Muncey J. P., Ridley R. M. Perceptual fading of a stabilized cortical image. Nature. 1971 Sep 17;233(5316):204–205. doi: 10.1038/233204a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Nachmias J. The orientation specificity of two visual after-effects. J Physiol. 1971 Feb;213(1):157–174. doi: 10.1113/jphysiol.1971.sp009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce P. R. Monocular fixation in human eye movement. Proc R Soc Lond B Biol Sci. 1967 Mar 28;167(1008):293–315. doi: 10.1098/rspb.1967.0028. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Kulikowski J. J. Orientational selectivity of the human visual system. J Physiol. 1966 Nov;187(2):437–445. doi: 10.1113/jphysiol.1966.sp008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITCHBURN R. W., FENDER D. H., MAYNE S. Vision with controlled movements of the retinal image. J Physiol. 1959 Jan 28;145(1):98–107. doi: 10.1113/jphysiol.1959.sp006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITCHBURN R. W., GINSBORG B. L. Vision with a stabilized retinal image. Nature. 1952 Jul 5;170(4314):36–37. doi: 10.1038/170036a0. [DOI] [PubMed] [Google Scholar]
- Ditchburn R. W., Foley-Fisher J. A. Assembled data in eye movements. Opt Acta (Lond) 1967 Apr;14(2):113–118. doi: 10.1080/713818024. [DOI] [PubMed] [Google Scholar]
- ENROTH C. The mechanism of flicker and fusion studied on single retinal elements in the dark-adapted eye of the cat. Acta Physiol Scand Suppl. 1952;27(100):1–67. [PubMed] [Google Scholar]
- Gerrits H. J., De Haan B., Vendrik A. J. Experiments with retinal stabilized images. Relations between the observations and neural data. Vision Res. 1966 Aug;6(7):427–440. doi: 10.1016/0042-6989(66)90051-4. [DOI] [PubMed] [Google Scholar]
- Gerrits H. J., Vendrik A. J. Artificial movements of a stabilized image. Vision Res. 1970 Dec;10(12):1443–1456. doi: 10.1016/0042-6989(70)90094-5. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D. M. Mislocation of test flashes during saccadic image displacements. Nature. 1970 Aug 15;227(5259):731–733. doi: 10.1038/227731a0. [DOI] [PubMed] [Google Scholar]
- Mackay D. M. Elevation of visual threshold by displacement of retinal image. Nature. 1970 Jan 3;225(5227):90–92. doi: 10.1038/225090a0. [DOI] [PubMed] [Google Scholar]
- NACHMIAS J. Two-dimensional motion of the retinal image during monocular fixation. J Opt Soc Am. 1959 Sep;49:901–908. doi: 10.1364/josa.49.000901. [DOI] [PubMed] [Google Scholar]
- Noda H., Freeman R. B., Jr, Gies B., Creutzfeldt O. D. Neuronal responses in the visual cortex of awake cats to stationary and moving targets. Exp Brain Res. 1971 May 26;112(4):389–405. doi: 10.1007/BF00234494. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. M., HERON W., HEBB D. O. Visual perception approached by the method of stabilized images. Can J Psychol. 1960 Jun;14:67–77. doi: 10.1037/h0083168. [DOI] [PubMed] [Google Scholar]
- RATLIFF F., RIGGS L. A. Involuntary motions of the eye during monocular fixation. J Exp Psychol. 1950 Dec;40(6):687–701. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- RIGGS L. A., RATLIFF F., CORNSWEET J. C., CORNSWEET T. N. The disappearance of steadily fixated visual test objects. J Opt Soc Am. 1953 Jun;43(6):495–501. doi: 10.1364/josa.43.000495. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Darian-Smith I., Bishop P. O. Binocular corresponding receptive fields of single units in the cat dorsal lateral geniculate nucleus. Vision Res. 1969 Oct;9(10):1297–1303. doi: 10.1016/0042-6989(69)90117-5. [DOI] [PubMed] [Google Scholar]
- Sharpe C. A fresh approach to stabilized retinal images. II. J Physiol. 1971;217 (Suppl):9P–10P. [PubMed] [Google Scholar]
- TOUSSAINT D., KUWABARA T., COGAN D. G. Retinal vascular patterns. II. Human retinal vessels studied in three dimensions. Arch Ophthalmol. 1961 Apr;65:575–581. doi: 10.1001/archopht.1961.01840020577022. [DOI] [PubMed] [Google Scholar]
- VOLKMANN F. C. Vision during voluntary saccadic eye movements. J Opt Soc Am. 1962 May;52:571–578. doi: 10.1364/josa.52.000571. [DOI] [PubMed] [Google Scholar]
- West D. C. Flicker and the stabilised retinal image. Vision Res. 1968 Jun;8(6):719–745. doi: 10.1016/0042-6989(68)90046-1. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H. Comparison of effects of eye movements and stimulus movements on striate cortex neurons of the monkey. J Neurophysiol. 1969 Nov;32(6):987–994. doi: 10.1152/jn.1969.32.6.987. [DOI] [PubMed] [Google Scholar]


