Abstract
1. Changes in the composition of foetal and maternal blood have been followed during the last 5-10 days of gestation and throughout parturition in the conscious sheep.
2. Catheters were placed in the foetal inferior vena cava through a tarsal vein and in a maternal uterine vein in ten ewes under sodium pentobarbitone anaesthesia. In four of the foetuses blood pressure and heart rates were recorded before and during parturition from an arterial catheter.
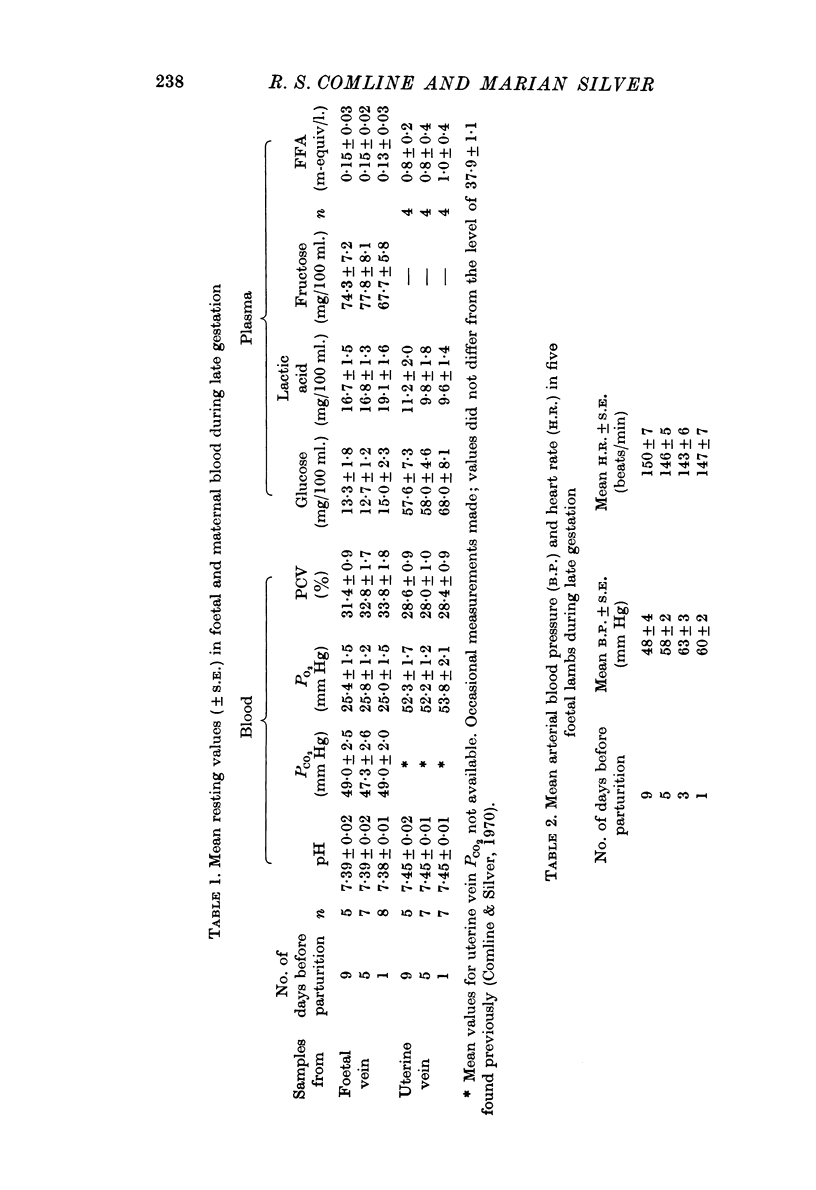
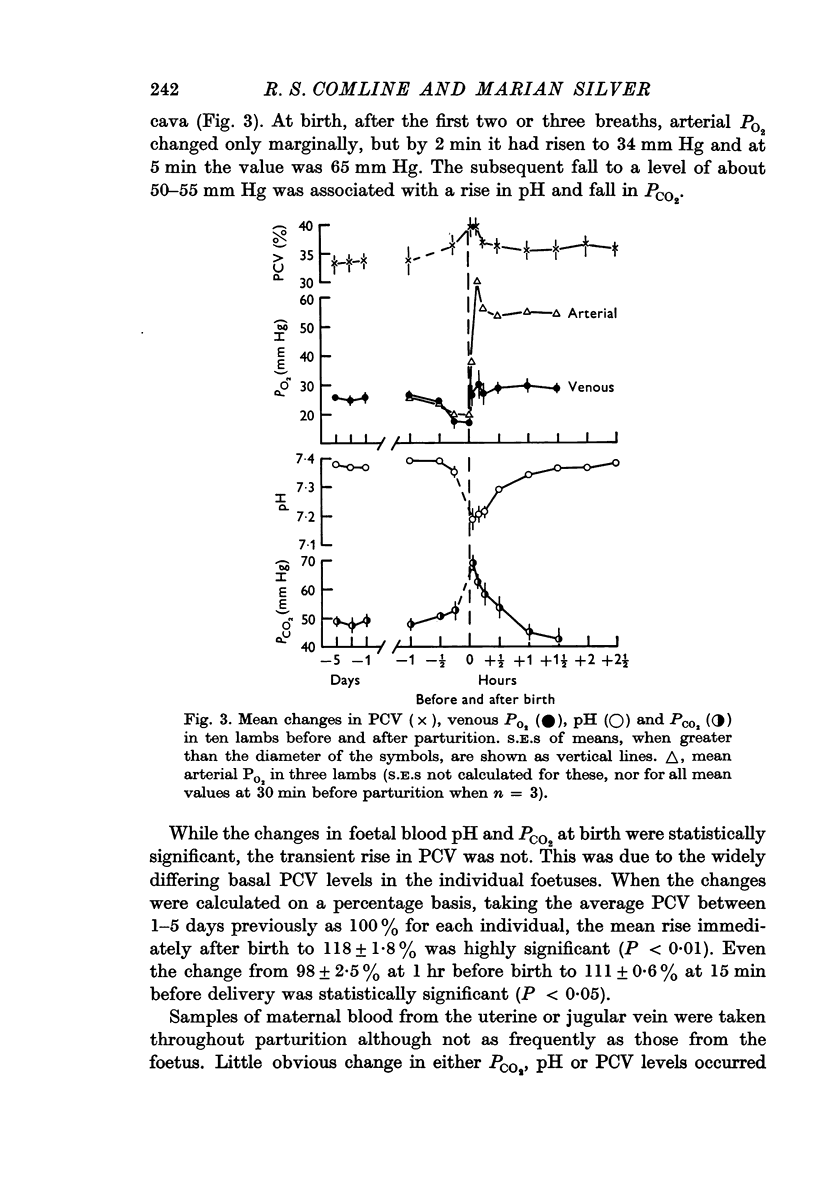
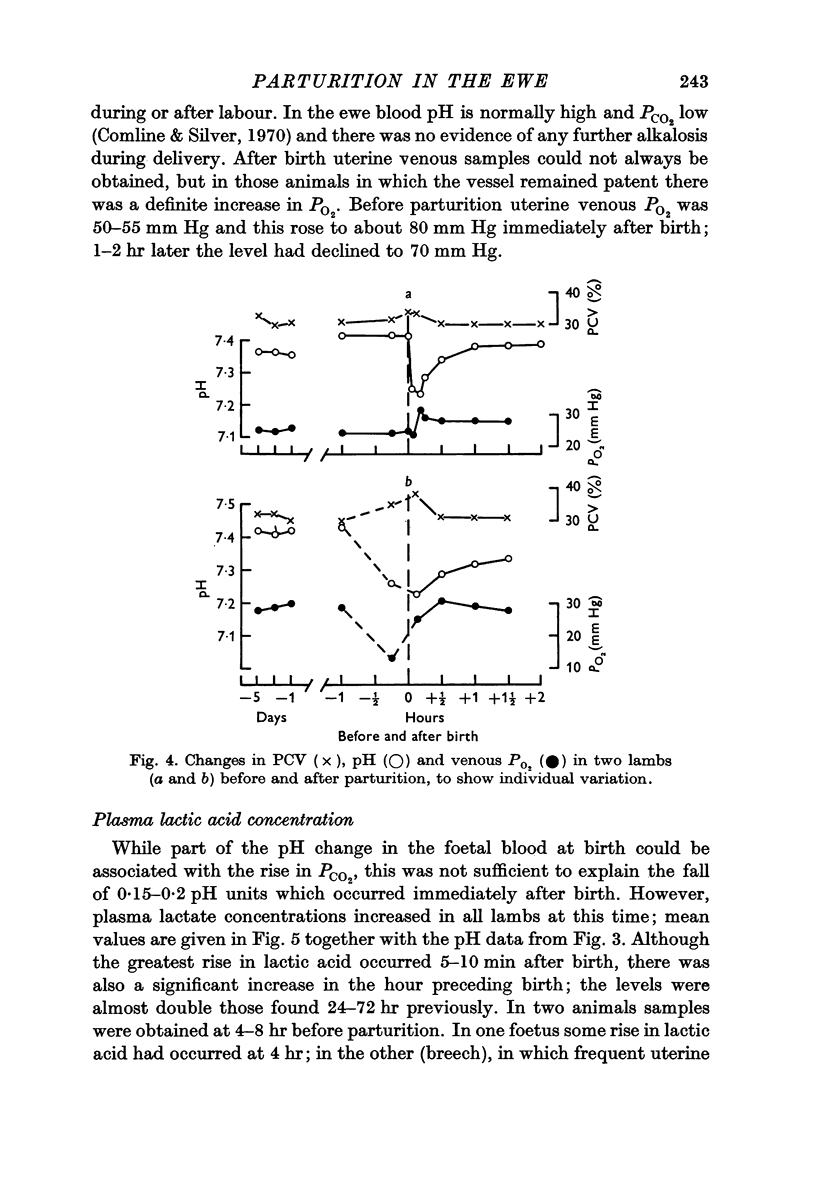
3. Foetal blood gas tensions, pH and PCV remained stable during the latter part of gestation and throughout labour until 15 min before delivery, when PO2 and pH fell while PCV and PCO2 rose in about 50% of the foetuses examined.
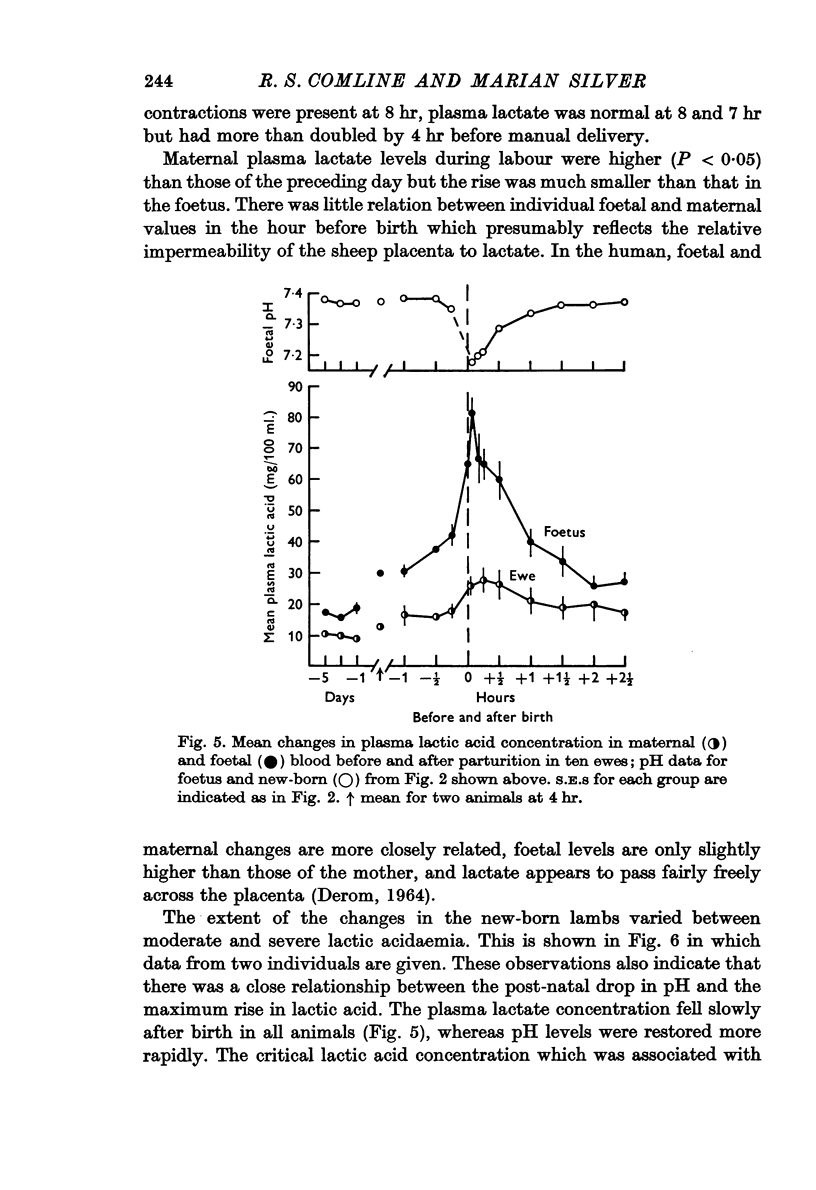
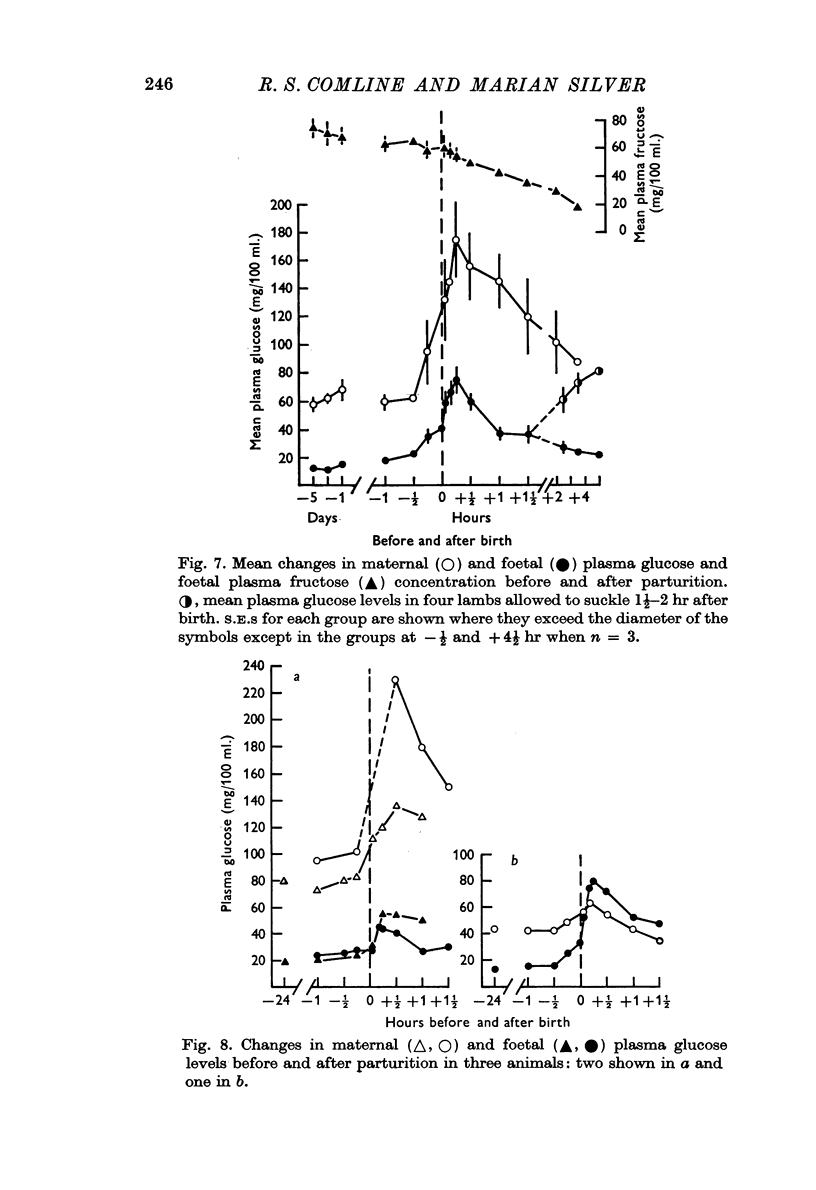
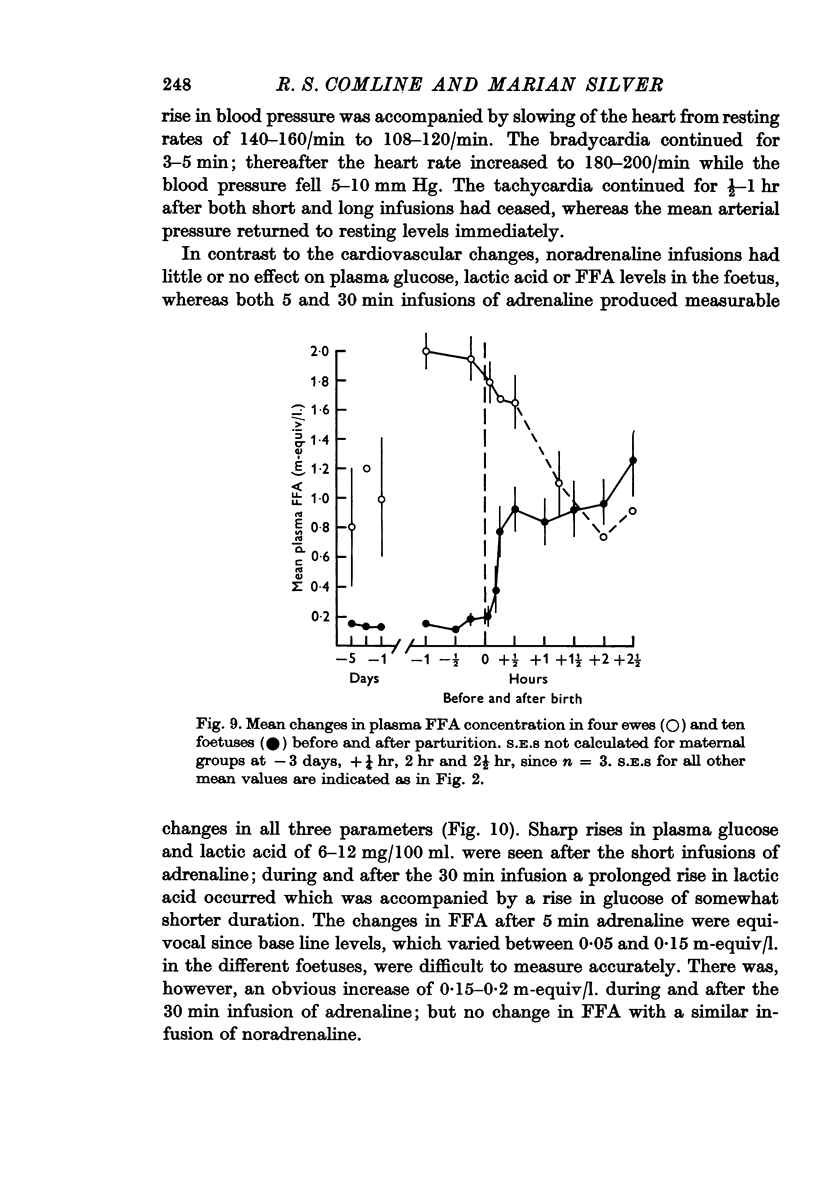
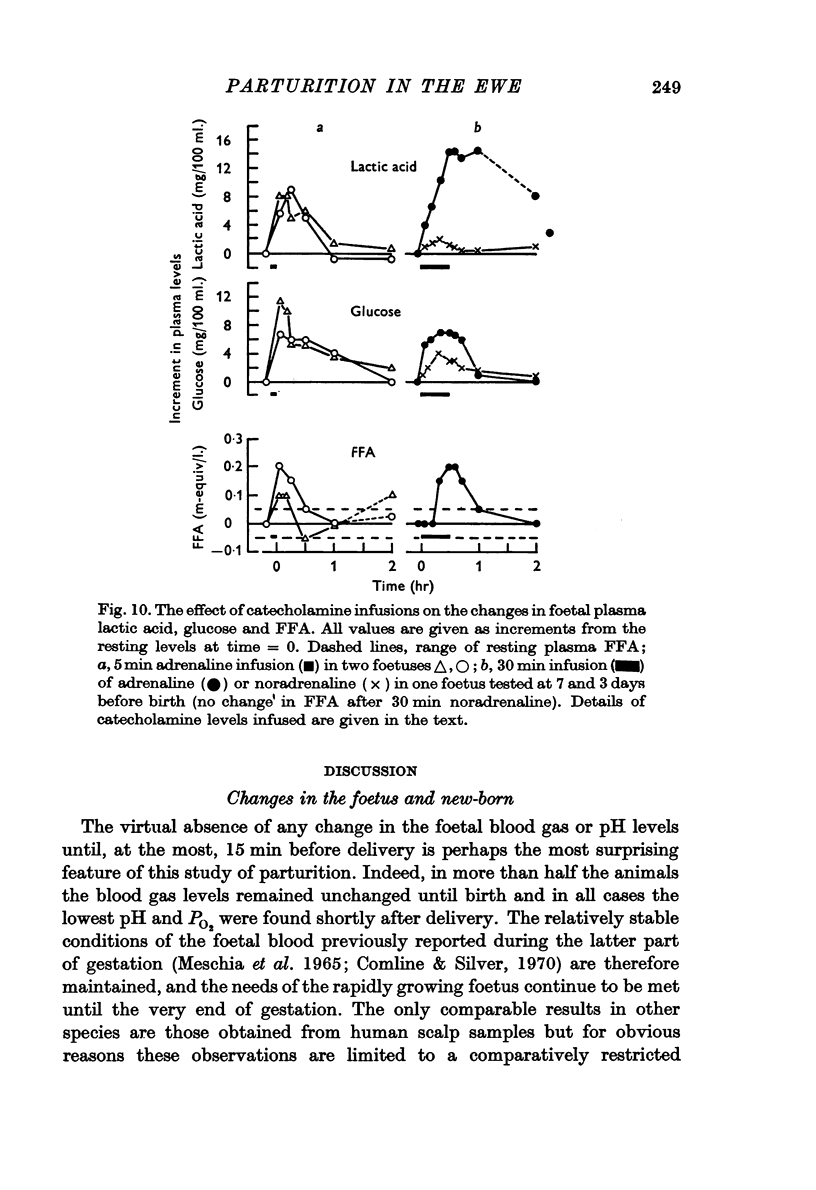
4. Metabolite levels were also relatively stable at the end of gestation. Plasma glucose in both maternal and foetal blood rose during the hour before birth, while foetal plasma lactate was elevated as early as 4 hr before birth and was unrelated to any maternal changes. Foetal fructose levels were maintained until after delivery.
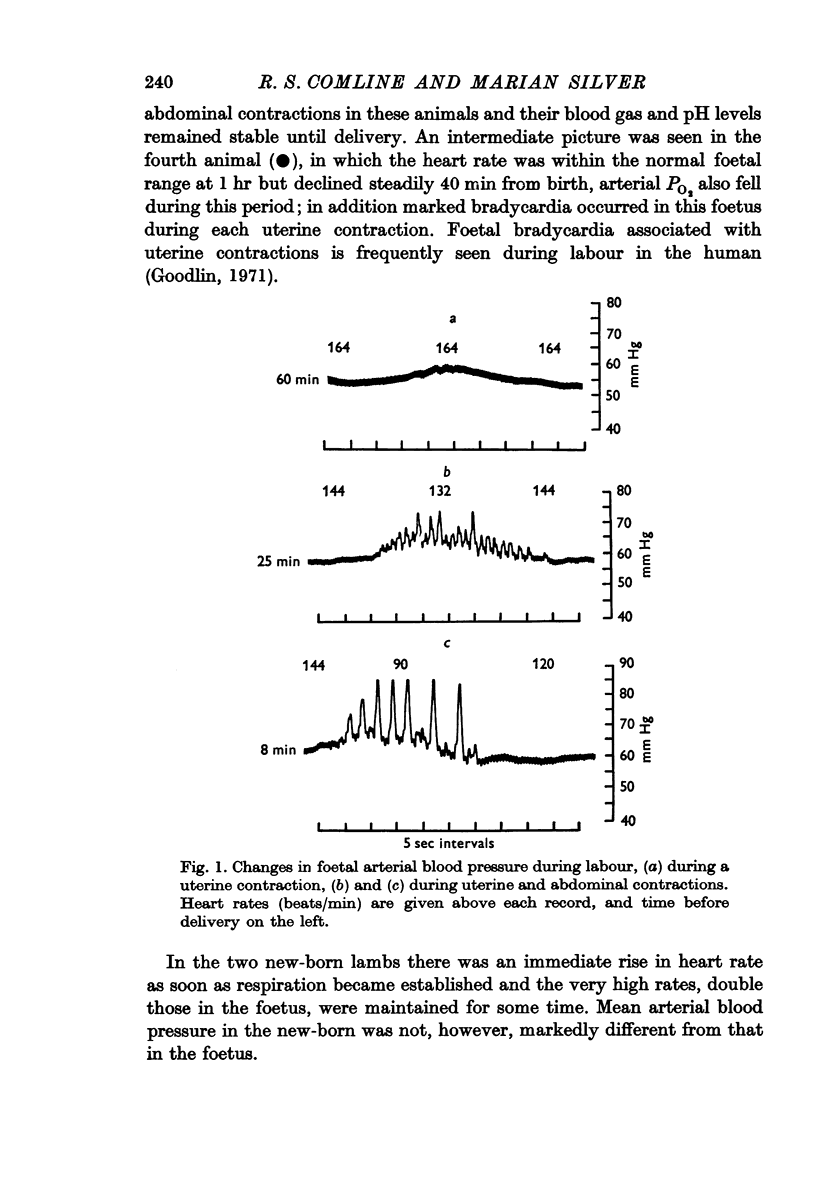
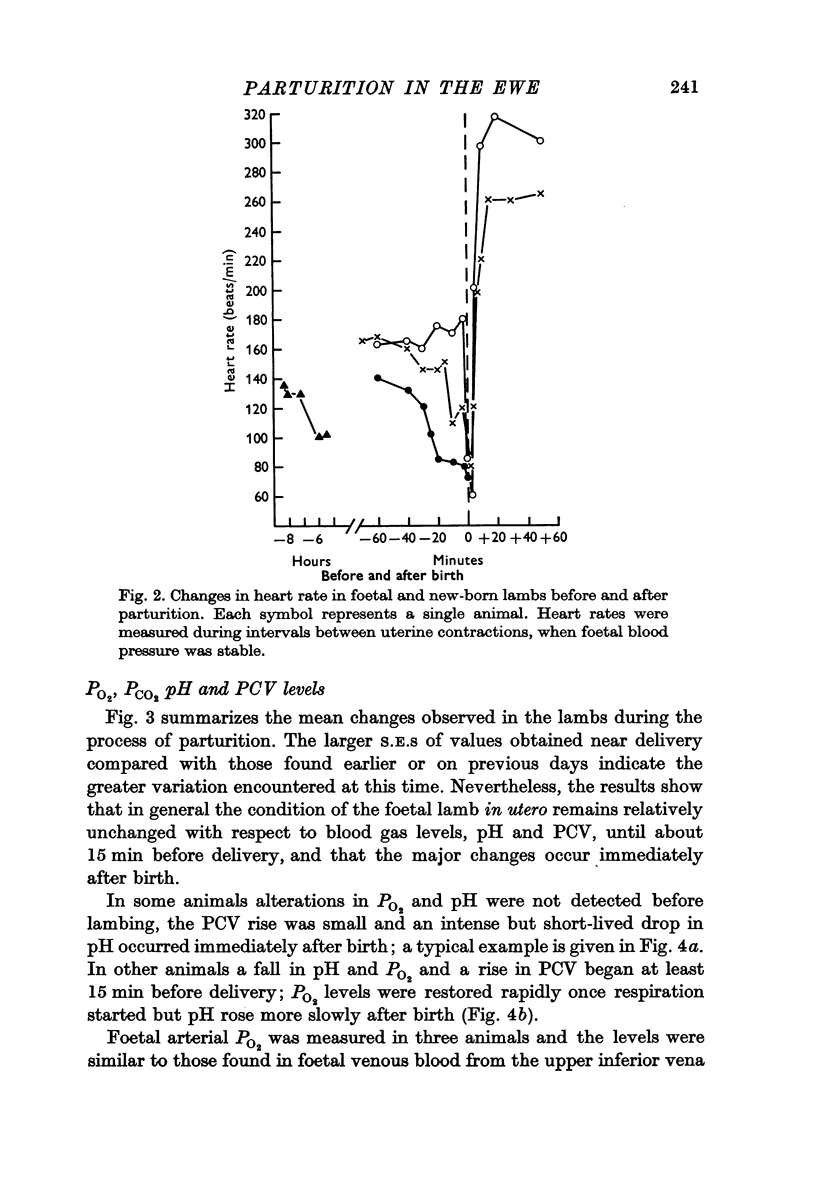
5. Rises in foetal blood pressure before birth were associated with uterine contractions. Foetal heart rate changes during labour varied in different individuals. The heart rate either fell gradually before birth or there was little change until a sudden drop at delivery.
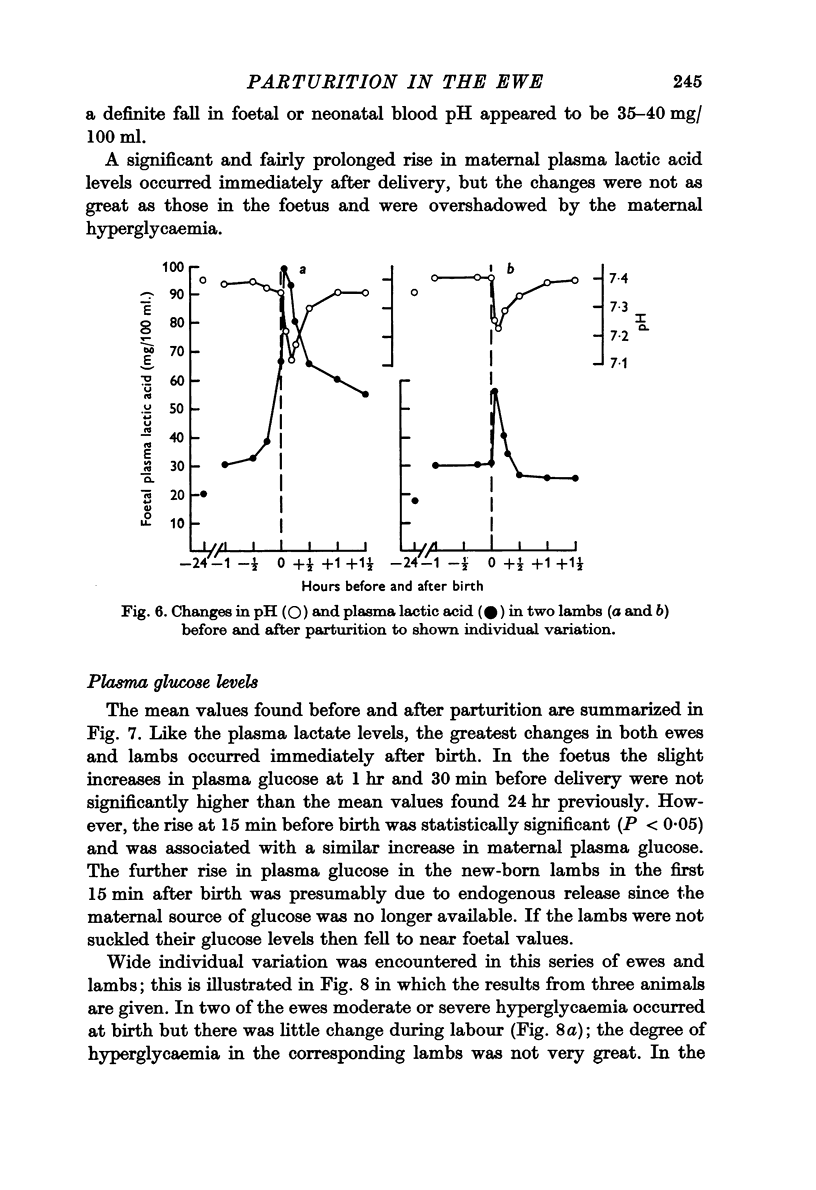
6. The most striking changes in the lamb occurred at, or a few minutes after, birth; pH and PO2 fell, PCO2 and PCV rose, and bradycardia at delivery was succeeded by prolonged tachycardia. There were marked increases in plasma glucose and lactic acid at this time.
7. PO2 rose rapidly once respiration was established, while pH and PCO2 levels were restored within ½-1 hr. Plasma FFA levels rose rapidly in the lambs 10-30 min after birth and remained high, while plasma glucose, lactate and fructose concentrations declined slowly in the 1-2 hr after birth, although suckling raised the plasma glucose levels. Considerable individual variation in the metabolite levels was found in both ewes and lambs.
8. In the majority of ewes delivery was associated with an abrupt maternal hyperglycaemia, with a much smaller rise in lactate and virtually no change in maternal blood gases or pH.
9. These findings are discussed in relation to existing information on new-born lambs and the human infant during birth.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G., Mills S. C. Free fatty acids and glucose in the plasma of newly born lambs: effects of environmental temperature. Biol Neonat. 1968;13(1):53–61. doi: 10.1159/000240132. [DOI] [PubMed] [Google Scholar]
- Alexander G., Mills S. C., Scott T. W. Changes in plasma glucose, lactate and free fatty acids in lambs during summit metabolism and treatment with catecholamines. J Physiol. 1968 Sep;198(2):277–289. doi: 10.1113/jphysiol.1968.sp008606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett J. M., Thorburn G. D. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol. 1969 Jun;44(2):285–286. doi: 10.1677/joe.0.0440285. [DOI] [PubMed] [Google Scholar]
- Bretscher J., Saling E. pH values in the human fetus during labor. Am J Obstet Gynecol. 1967 Apr 1;97(7):906–911. doi: 10.1016/0002-9378(67)90515-7. [DOI] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER I. A., SILVER M. FACTORS RESPONSIBLE FOR THE STIMULATION OF THE ADRENAL MEDULLA DURING ASPHYXIA IN THE FOETAL LAMB. J Physiol. 1965 May;178:211–238. doi: 10.1113/jphysiol.1965.sp007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comline R. S., Nathanielsz P. W., Paisey R. B., Silver M. Cortisol turnover in the sheep foetus immediately prior to parturition. J Physiol. 1970 Sep;210(2):141P–142P. [PubMed] [Google Scholar]
- Comline R. S., Silver M. Daily changes in foetal and maternal blood of conscious pregnant ewes, with catheters in umbilical and uterine vessels. J Physiol. 1970 Aug;209(3):567–586. doi: 10.1113/jphysiol.1970.sp009180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWKINS M. J. CHANGES IN BLOOD GLUCOSE AND NON-ESTERIFIED FATTY ACIDS IN THE FOETAL AND NEWBORN LAMB AFTER INJECTION OF ADRENALINE. Biol Neonat. 1964;7:160–166. doi: 10.1159/000239919. [DOI] [PubMed] [Google Scholar]
- DAWKINS M. J., HULL D. BROWN ADIPOSE TISSUE AND THE RESPONSE OF NEW-BORN RABBITS TO COLD. J Physiol. 1964 Aug;172:216–238. doi: 10.1113/jphysiol.1964.sp007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEROM R. ANAEROBIC METABOLISM IN THE HUMAN FETUS. 1. THE NORMAL DELIVERY. Am J Obstet Gynecol. 1964 May 15;89:241–251. doi: 10.1016/0002-9378(64)90717-3. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. D., Robinson B. H. An analysis of the reflex systemic vasodilator response elicited by lung inflation in the dog. J Physiol. 1968 Mar;195(2):387–406. doi: 10.1113/jphysiol.1968.sp008464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Duncan S. L., Lewis B. V., Merlet C. L., Owen-Thomas J. B., Reeves J. T. Hypoxaemia and aortic chemoreceptor function in foetal lambs. J Physiol. 1969 Mar;201(1):105–116. doi: 10.1113/jphysiol.1969.sp008745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gare D. J., Shime J., Paul W. M., Hoskins M. Oxygen administration during labor. Am J Obstet Gynecol. 1969 Nov 15;105(6):954–961. doi: 10.1016/0002-9378(69)90104-5. [DOI] [PubMed] [Google Scholar]
- Goodlin R. C. Intrapartum fetal heart rate responses and plethysmographic pulse. Am J Obstet Gynecol. 1971 May 15;110(2):210–226. doi: 10.1016/0002-9378(71)90608-9. [DOI] [PubMed] [Google Scholar]
- Goodwin J. W., Paul W. M. Umbilical arterial oxygen tension of the fetal lamb in utero. Effects of oxytocic drugs. Am J Obstet Gynecol. 1966 Oct 1;96(3):362–366. doi: 10.1016/0002-9378(66)90239-0. [DOI] [PubMed] [Google Scholar]
- Heim T., Hull D. The blood flow and oxygen consumption of brown adipose tissue in the new-born rabbit. J Physiol. 1966 Sep;186(1):42–55. doi: 10.1113/jphysiol.1966.sp008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson J. C., Schofield B. M., Turner C. B., Wolff H. S. Parturition in the sheep. J Physiol. 1965 Dec;181(3):560–567. doi: 10.1113/jphysiol.1965.sp007782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES L. S. Acidosis of the newborn and its relation to birth asphyxia. Acta Paediatr Suppl. 1960 Mar;49(Suppl 122):17–28. doi: 10.1111/j.1651-2227.1960.tb05962.x. [DOI] [PubMed] [Google Scholar]
- James J. E., Daly M. de B. Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. J Physiol. 1969 Mar;201(1):87–104. doi: 10.1113/jphysiol.1969.sp008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Wendel H. Adjustment of arterial blood gases and acid base balance in the normal newborn infant during the first week of life. Biol Neonat. 1968;12(3):136–161. doi: 10.1159/000240100. [DOI] [PubMed] [Google Scholar]
- Low J. A., Boston R. W., Cervenko F. W. Effect of low maternal carbon dioxide tension on placental gas exchange. Am J Obstet Gynecol. 1970 Apr 1;106(7):1032–1043. doi: 10.1016/s0002-9378(16)34089-3. [DOI] [PubMed] [Google Scholar]
- MESCHIA G., COTTER J. R., BREATHNACH C. S., BARRON D. H. THE HEMOGLOBIN, OXYGEN, CARBON DIOXIDE AND HYDROGEN ION CONCENTRATIONS IN THE UMBILICAL BLOODS OF SHEEP AND GOATS AS SAMPLED VIA INDWELLING PLASTIC CATHETERS. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:185–195. doi: 10.1113/expphysiol.1965.sp001780. [DOI] [PubMed] [Google Scholar]
- Roux J. F., Romney S. L. Plasma free fatty acids and glucose concentrations in the human fetus and newborn exposed to various environmental conditions. Am J Obstet Gynecol. 1967 Jan 15;97(2):268–276. doi: 10.1016/0002-9378(67)90550-9. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- VANDUYNE C., PARKER H. R., HOLM L. W. METABOLISM OF FREE FATTY ACIDS DURING PERINATAL LIFE OF LAMBS. Am J Obstet Gynecol. 1965 Jan 15;91:277–285. doi: 10.1016/0002-9378(65)90212-7. [DOI] [PubMed] [Google Scholar]
- Walker A., Phillips L., Powe L., Wood C. A new instrument for the measurement of tissue pO2 of human fetal scalp. Am J Obstet Gynecol. 1968 Jan 1;100(1):63–71. doi: 10.1016/s0002-9378(15)33638-3. [DOI] [PubMed] [Google Scholar]


