Abstract
1. Human subjects were asked to rate both blanks and very dim flashes of light under conditions of complete dark adaptation at 7° in the periphery. The ratings used were 0, 1, 2, 3, 4, 5, and 6.
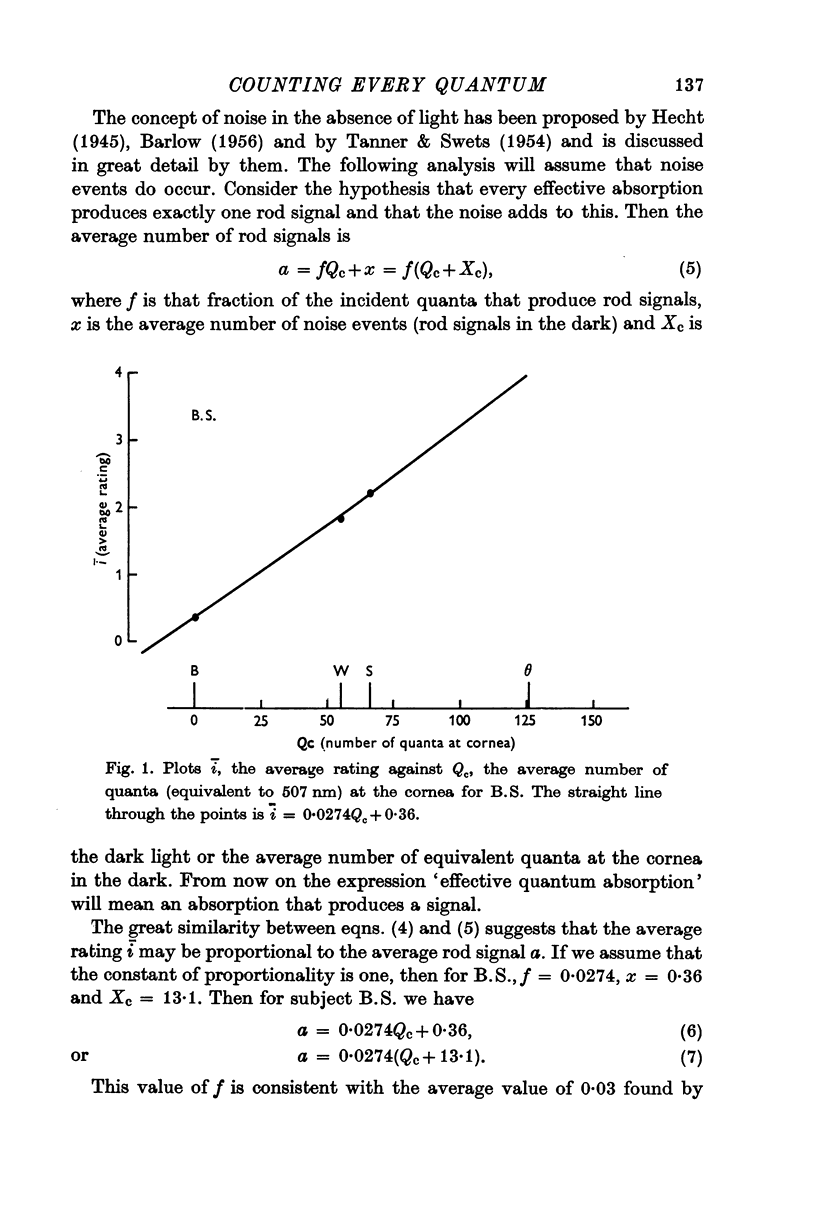
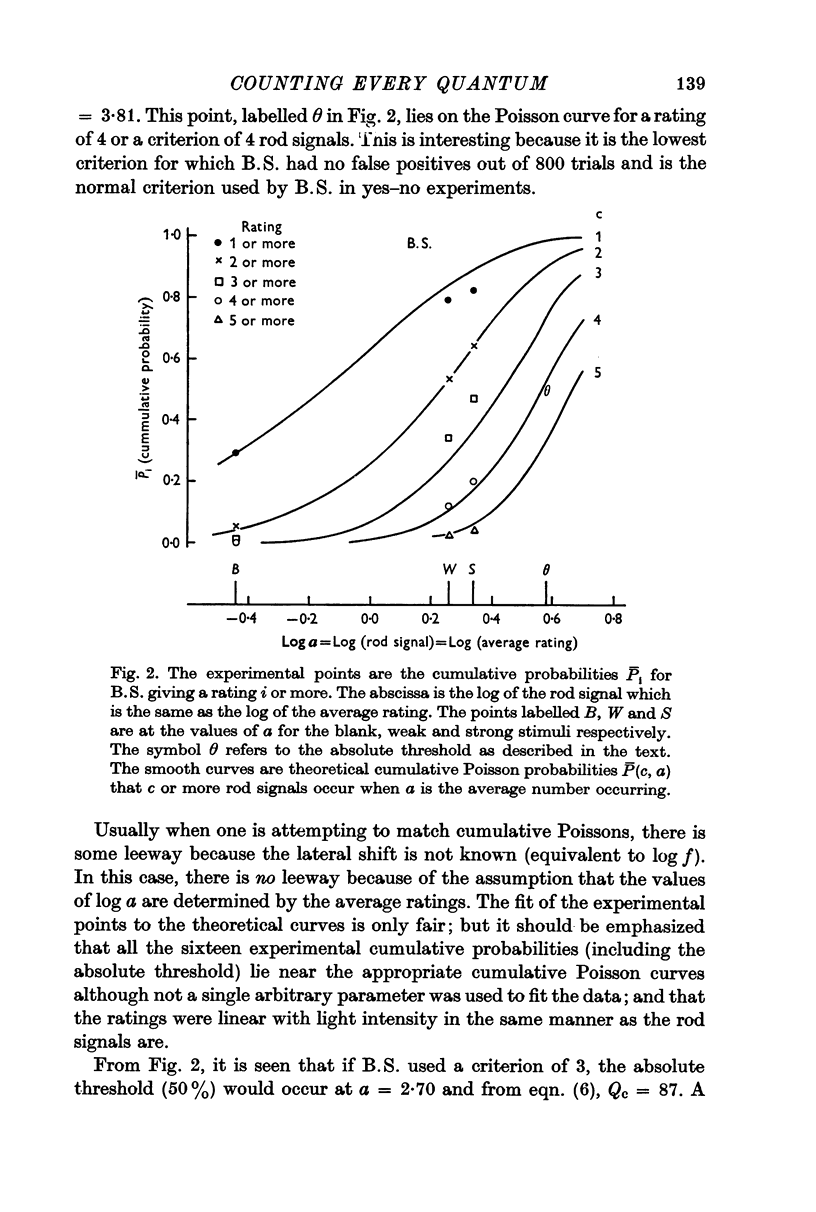
2. For one subject (B.S.) the distributions of ratings were approximately Poisson distributions. The data were consistent with each rating being the actual number of effective quantal absorptions plus the number of noise events. This subject was presumably able to count every rod signal (effective absorptions plus noise).
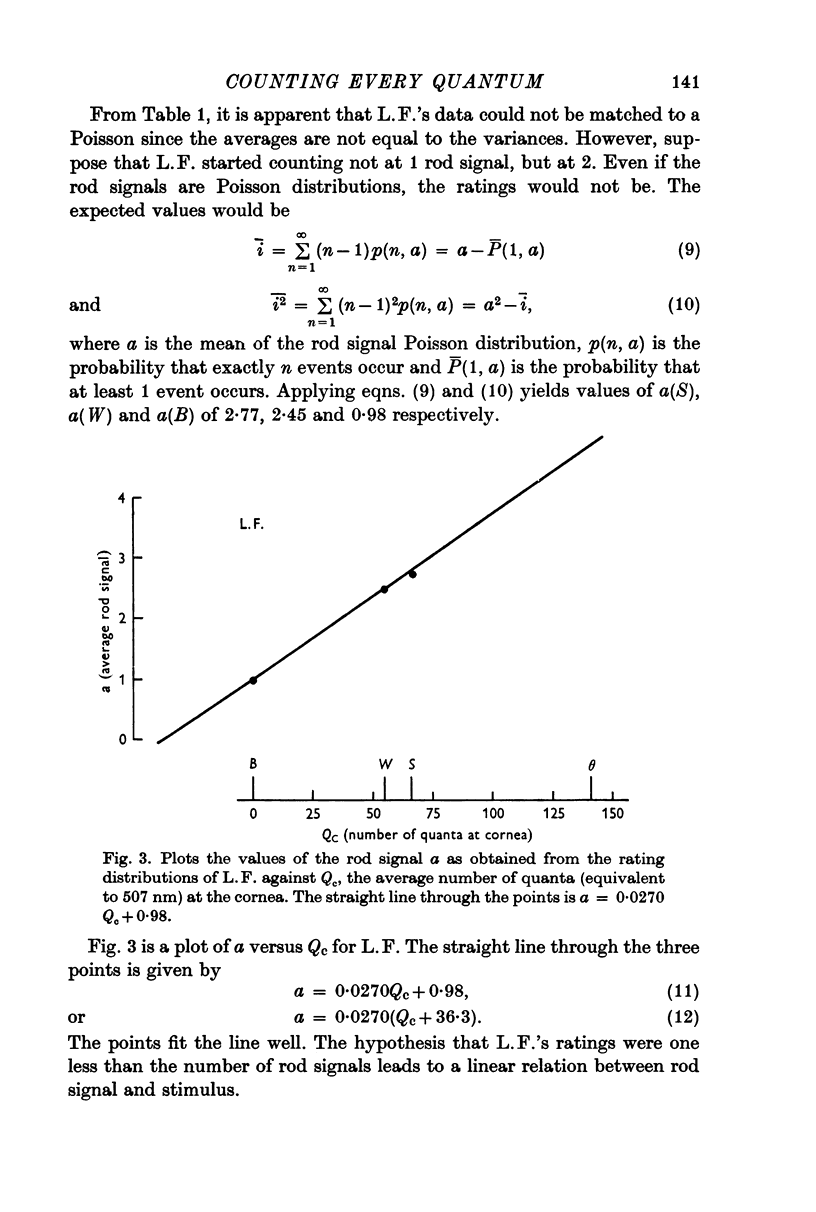
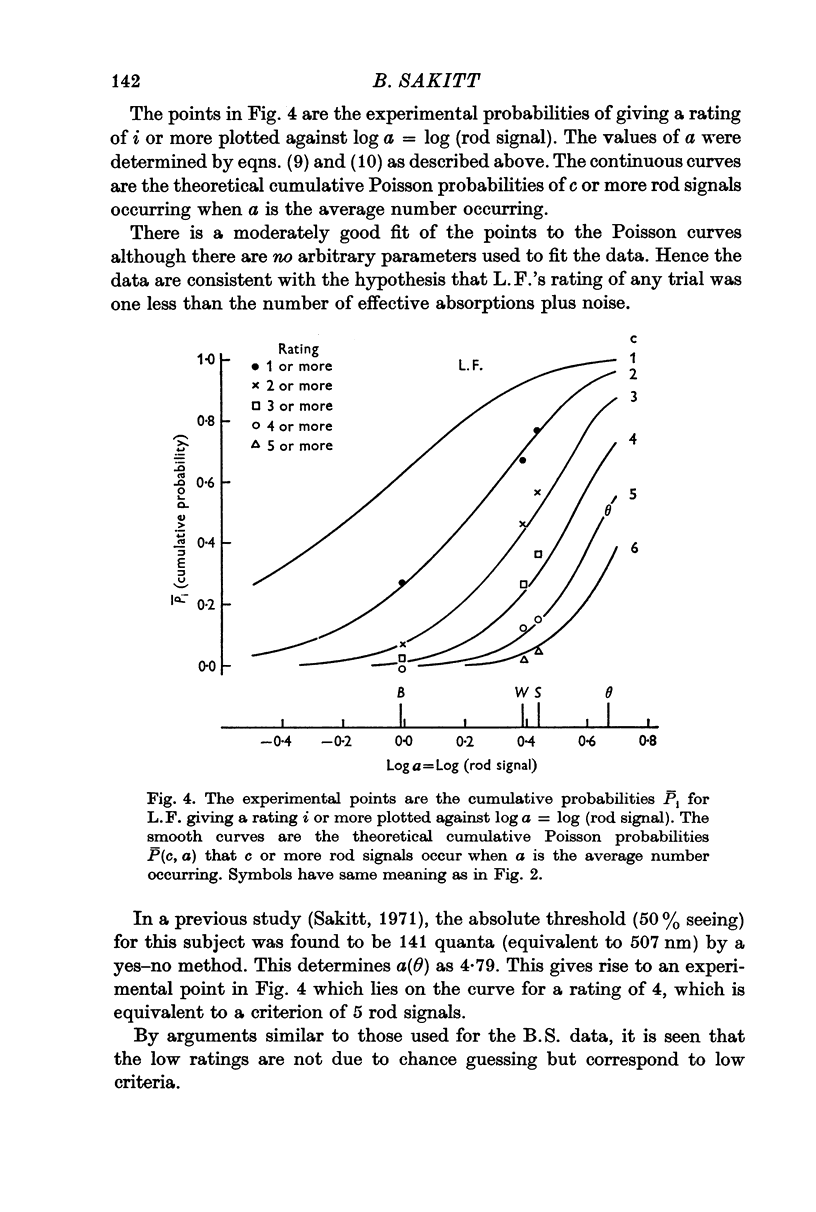
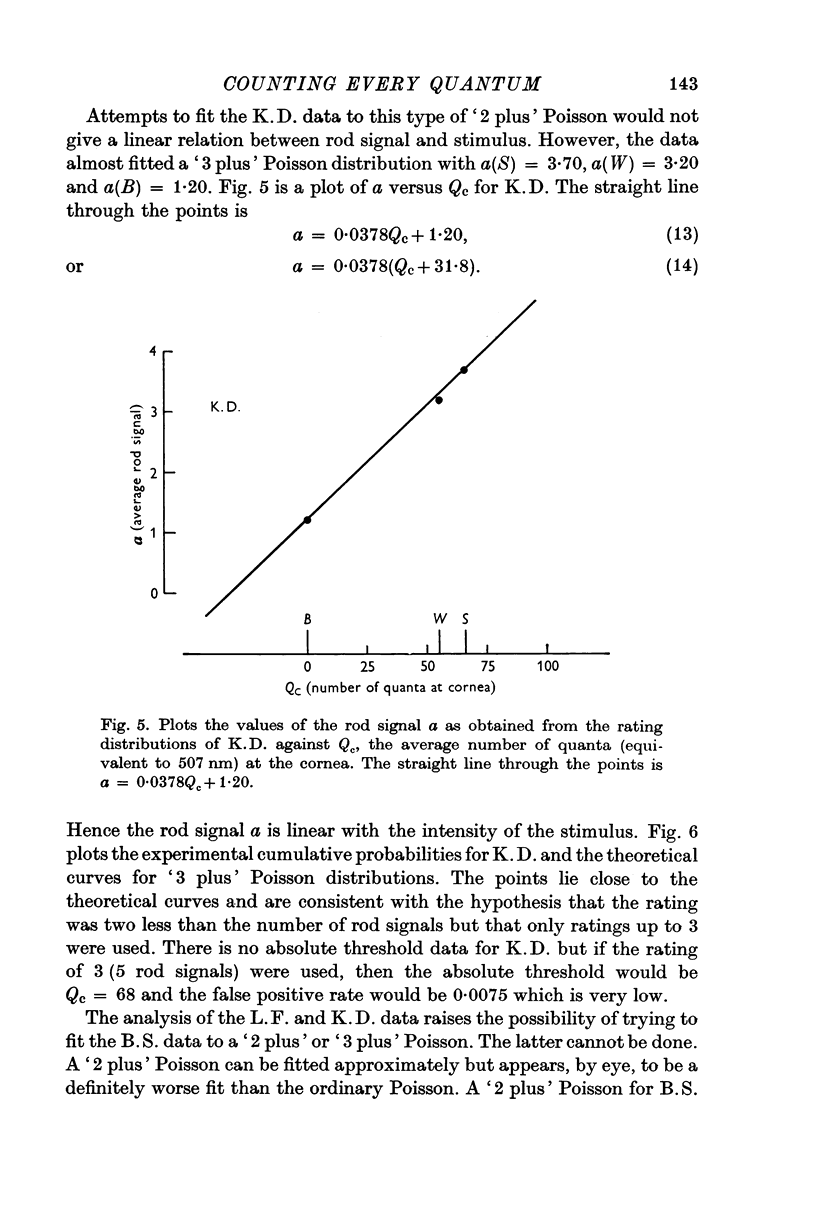
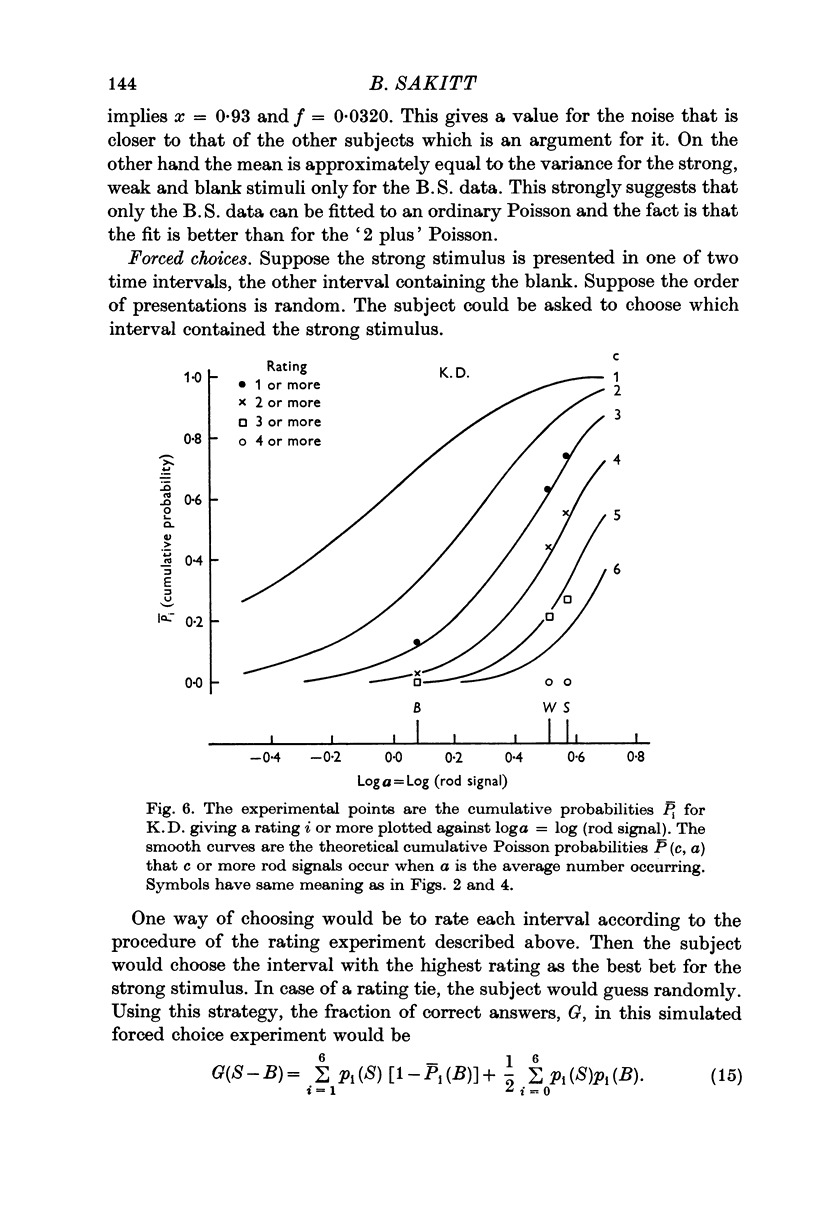
3. For two other subjects, the data were consistent with the ratings being one less (L.F.) and two less (K.D.) than the number of effective absorptions plus noise. They were able to count every rod signal beginning with 2 and 3 respectively. A fourth subject's erratic data could not be fitted.
4. The fraction of quanta incident at the cornea that resulted in a rod signal was estimated to be about 0·03 which is consistent with physical estimates of effective absorption for that retinal region.
5. A simulated forced choice experiment leads to an absolute threshold about 0·40 log units below the normal yes-no absolute threshold. This and other results indicate that subjects can use the sensory information they receive even when only 1, 2 or 3 quanta are effectively absorbed, depending on the individual. Humans may be able to count every action potential or every discrete burst of action potentials in some critical neurone.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Retinal noise and absolute threshold. J Opt Soc Am. 1956 Aug;46(8):634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;Suppl 3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Barlow H. B. Measurements of the quantum efficiency of discrimination in human scotopic vision. J Physiol. 1962 Jan;160(1):169–188. doi: 10.1113/jphysiol.1962.sp006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGINS W. A. The quantum efficiency of bleaching of rhodopsin in situ. J Physiol. 1955 Jul 28;129(1):22–3P. [PubMed] [Google Scholar]
- NACHMIAS J., STEINMAN R. M. STUDY OF ABSOLUTE VISUAL DETECTION BY THE RATING-SCALE METHOD. J Opt Soc Am. 1963 Oct;53:1206–1206. doi: 10.1364/josa.53.001206. [DOI] [PubMed] [Google Scholar]
- PIRENNE M. H., MARRIOTT F. H. Absolute threshold and frequency-of-seeing curves. J Opt Soc Am. 1955 Nov;45(11):909–912. doi: 10.1364/josa.45.000909. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. The difference spectrum and the photosensitivity of rhodopsin in the living human eye. J Physiol. 1956 Oct 29;134(1):11–29. doi: 10.1113/jphysiol.1956.sp005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. The rhodopsin density in the human rods. J Physiol. 1956 Oct 29;134(1):30–46. doi: 10.1113/jphysiol.1956.sp005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitt B. Configuration dependence of scotopic spatial summation. J Physiol. 1971 Aug;216(3):513–529. doi: 10.1113/jphysiol.1971.sp009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANNER W. P., Jr, SWETS J. A. A decision-making theory of visual detection. Psychol Rev. 1954 Nov;61(6):401–409. doi: 10.1037/h0058700. [DOI] [PubMed] [Google Scholar]
- Westheimer G. The Maxwellian view. Vision Res. 1966 Dec;6(12):669–682. doi: 10.1016/0042-6989(66)90078-2. [DOI] [PubMed] [Google Scholar]


