Abstract
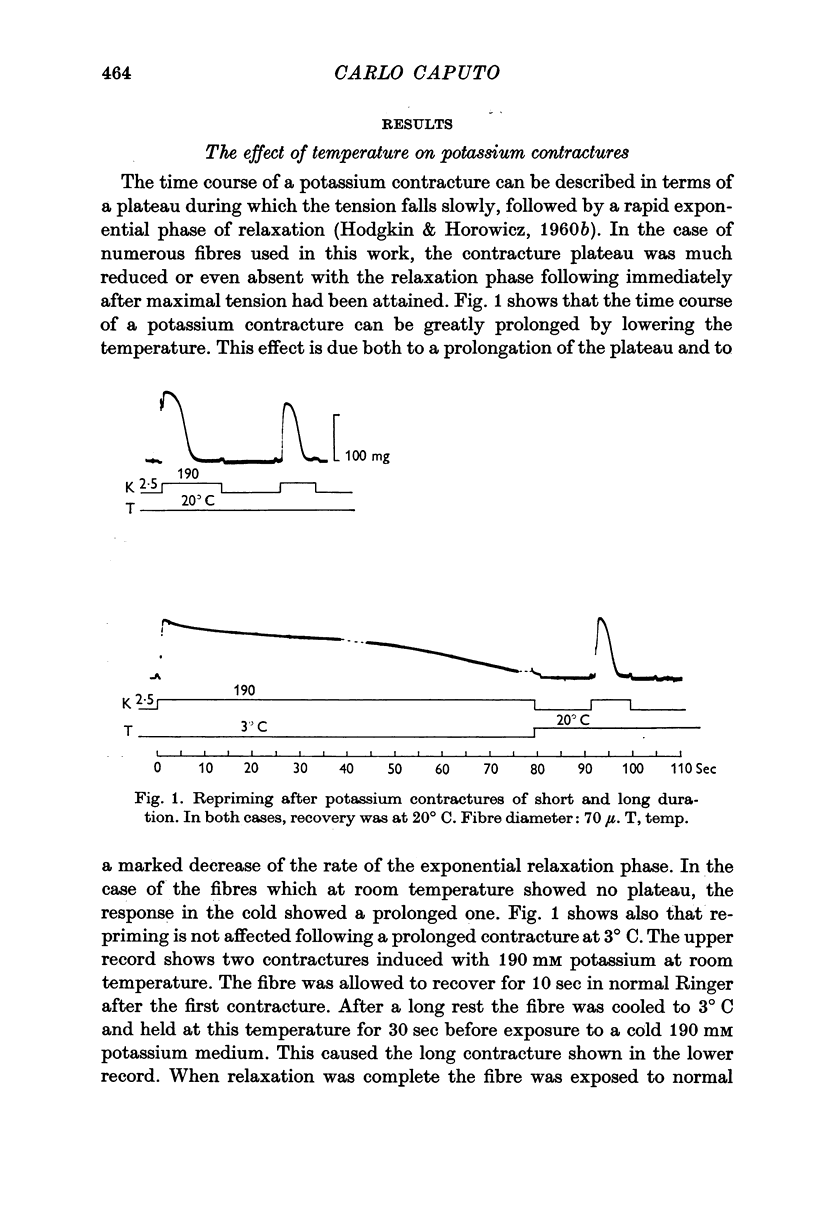
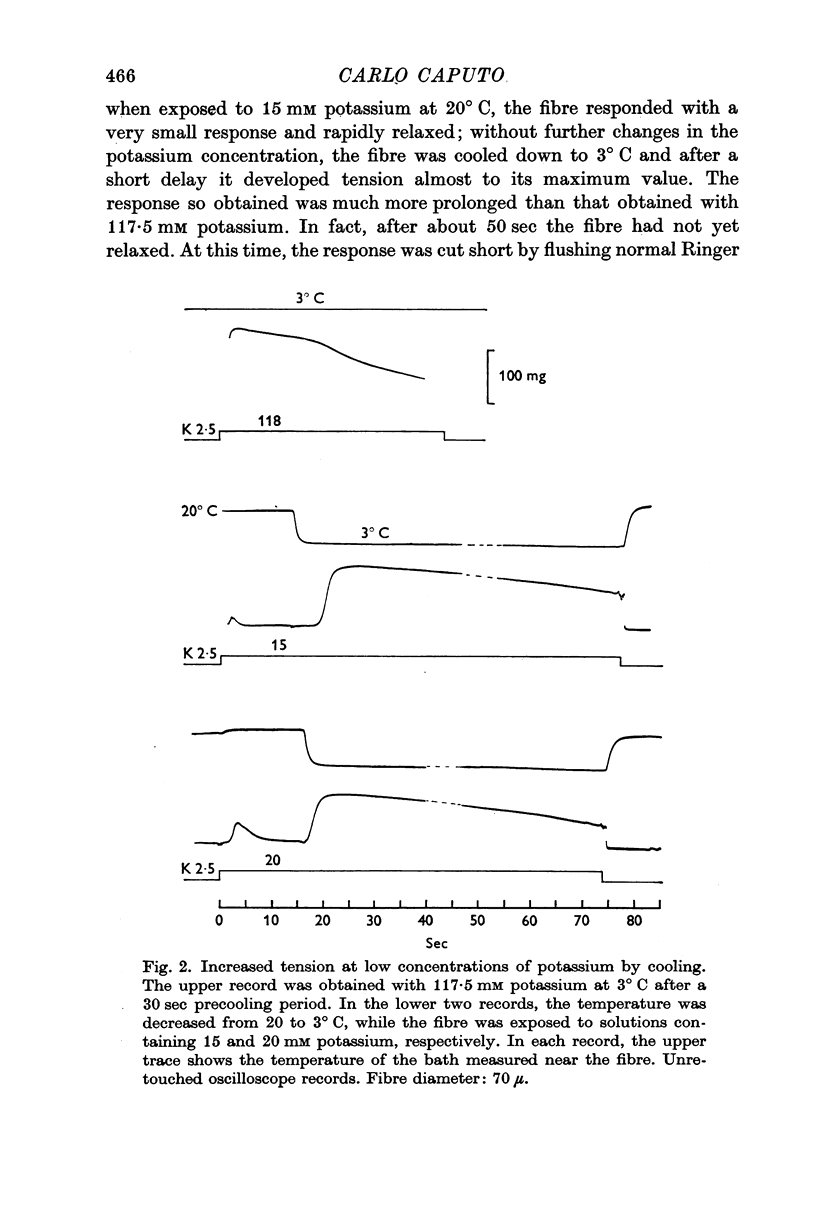
1. Potassium contractures are affected by low temperature: the maximum contracture tension is diminished by about 15% at 3° C, while the response time course is greatly prolonged.
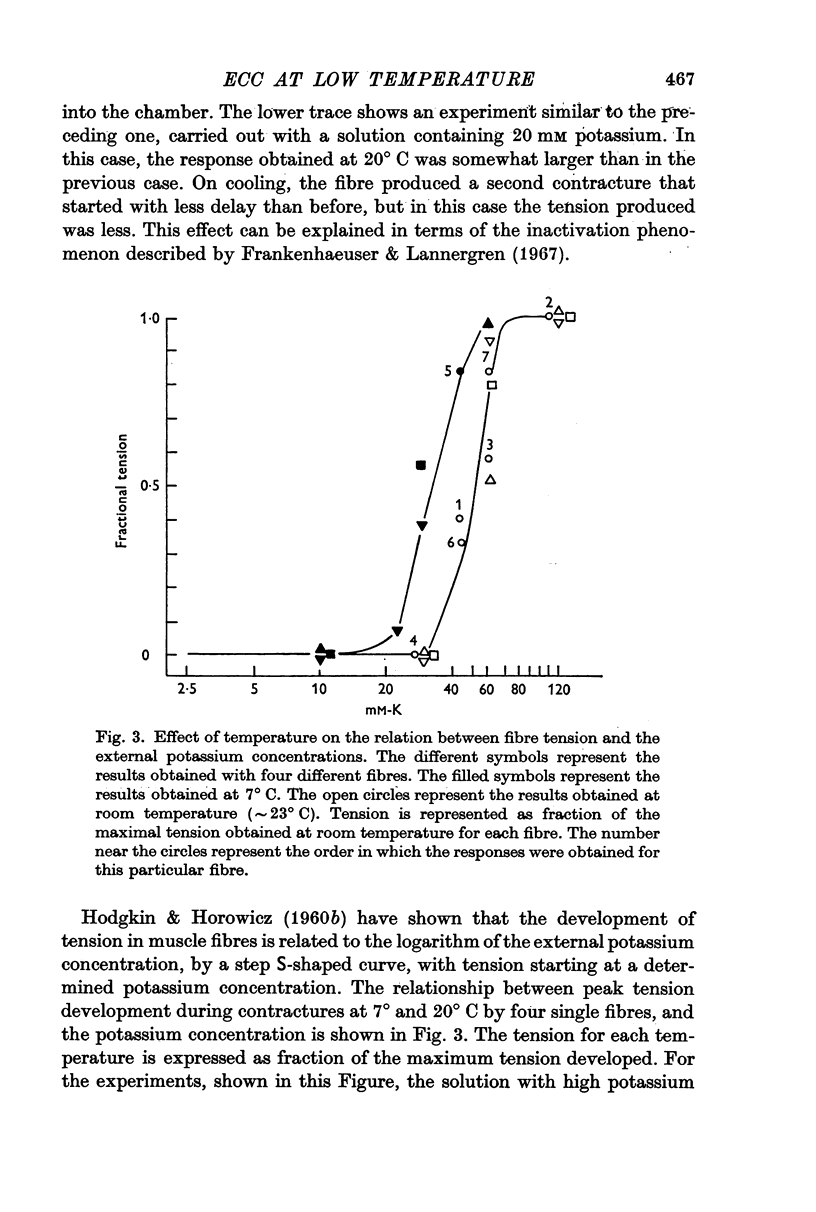
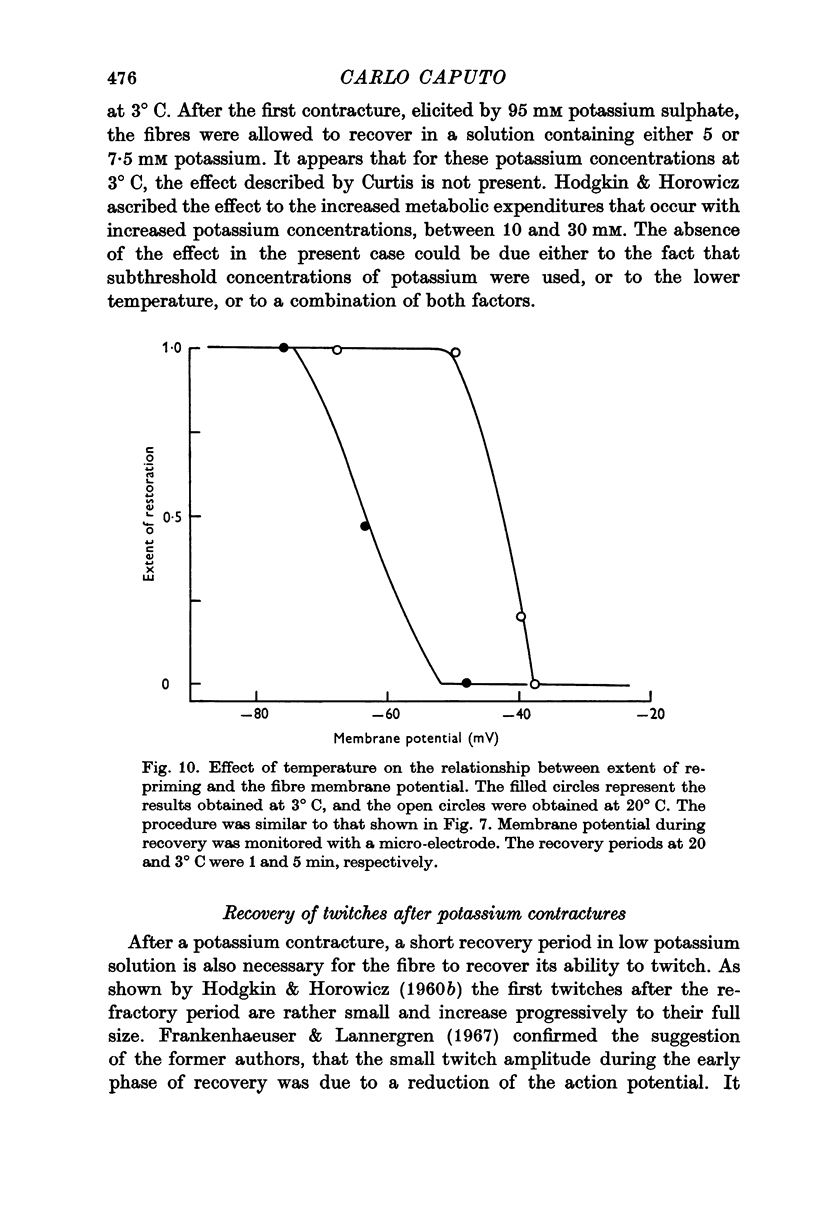
2. The contractile threshold for potassium contractures is lowered by about 10 mV at 3° C.
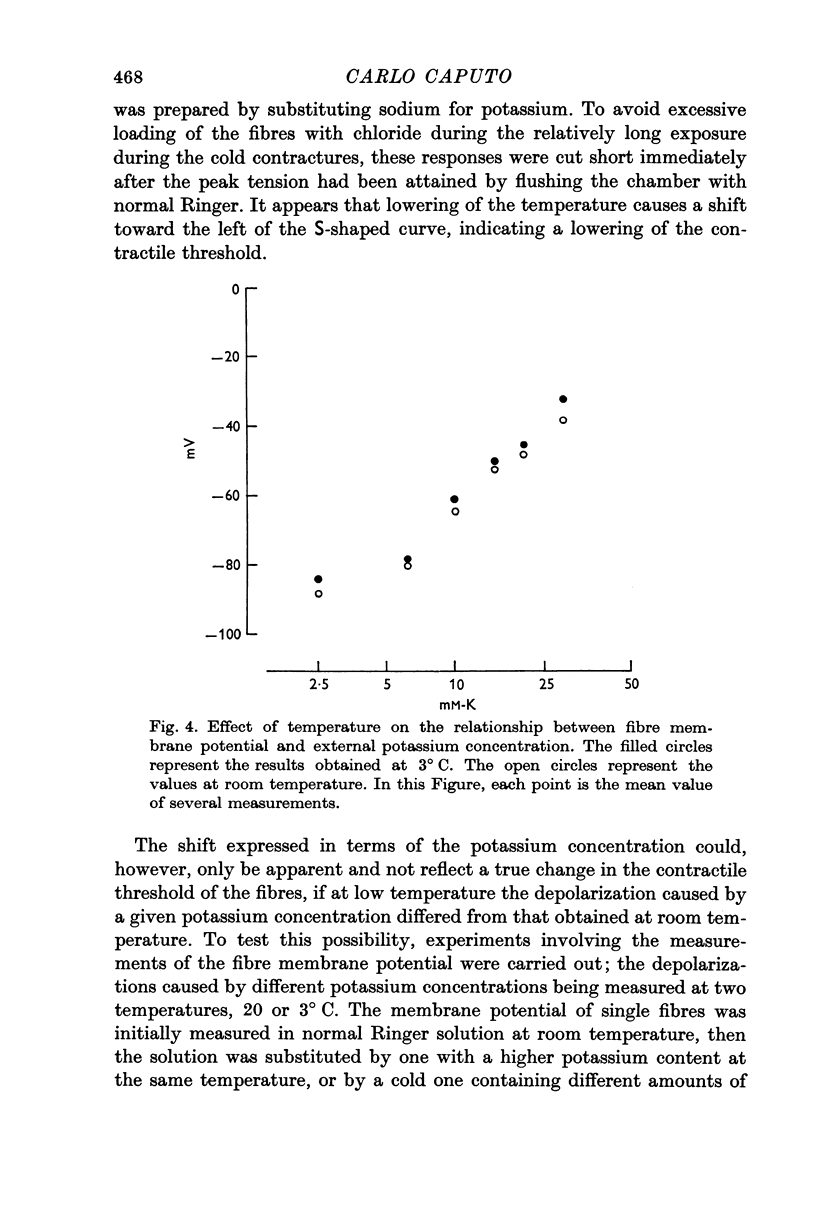
3. The fibre's membrane is depolarized by approximately the same amount when exposed to solutions with increased potassium concentrations at 20 or 3° C.
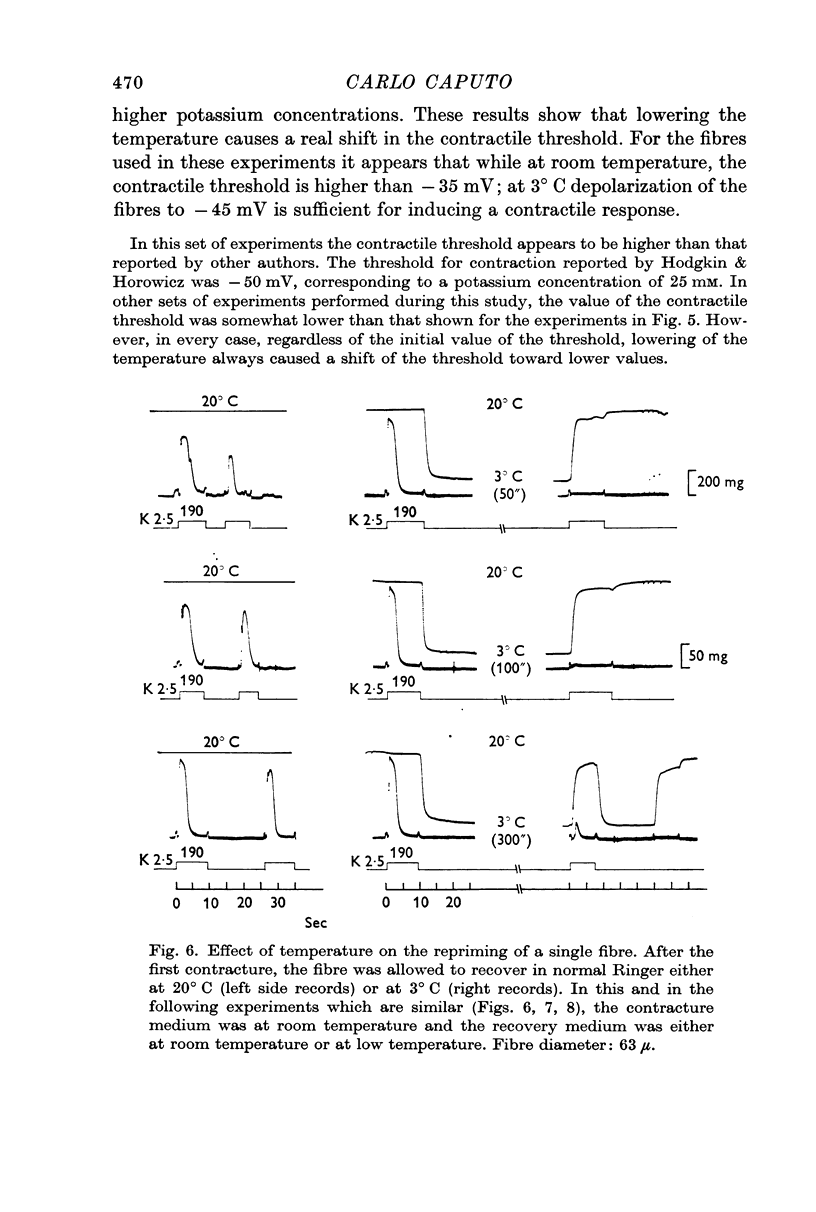
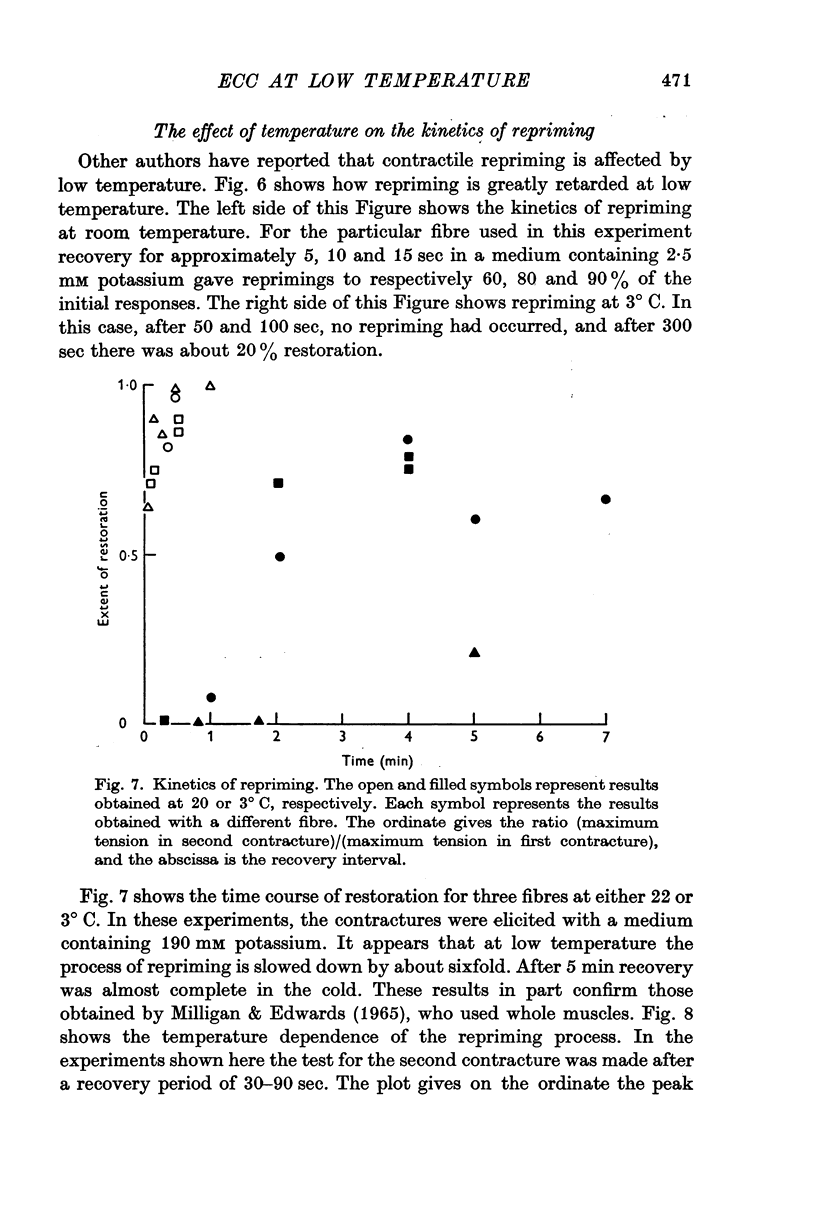
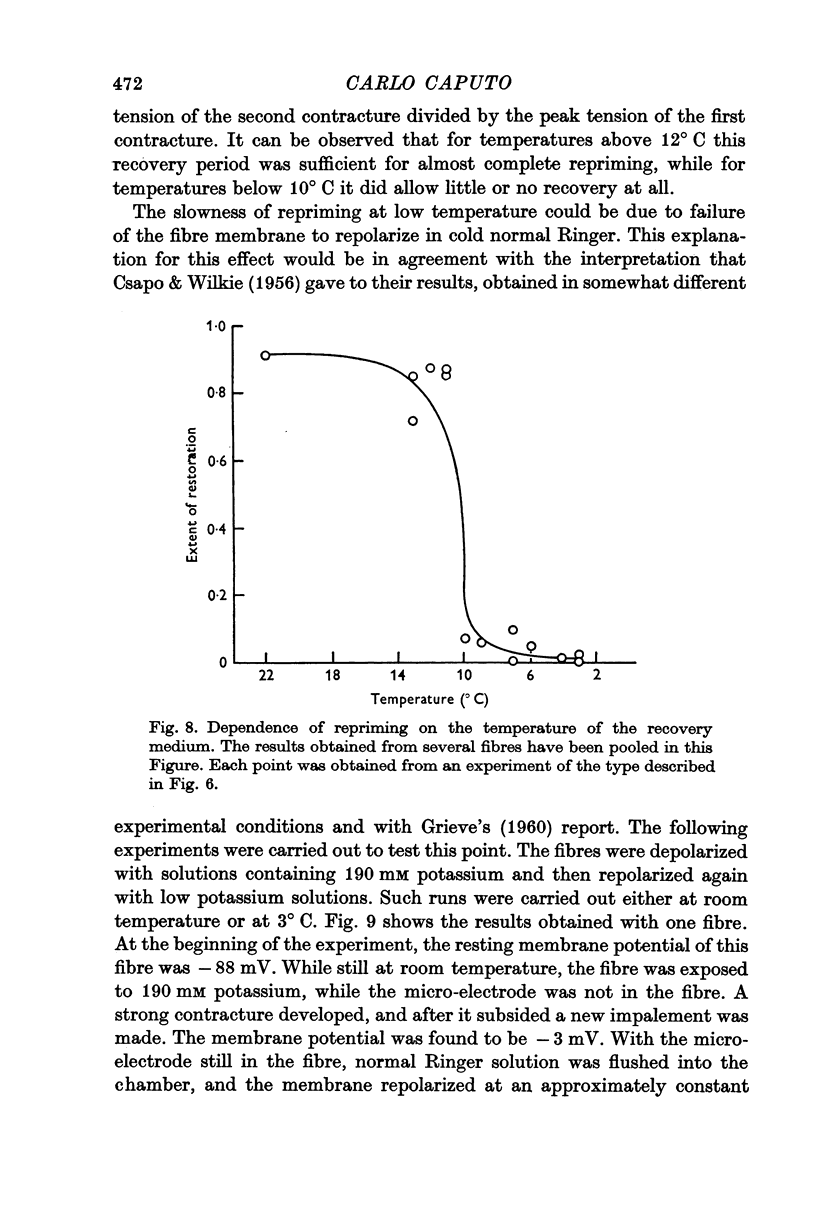
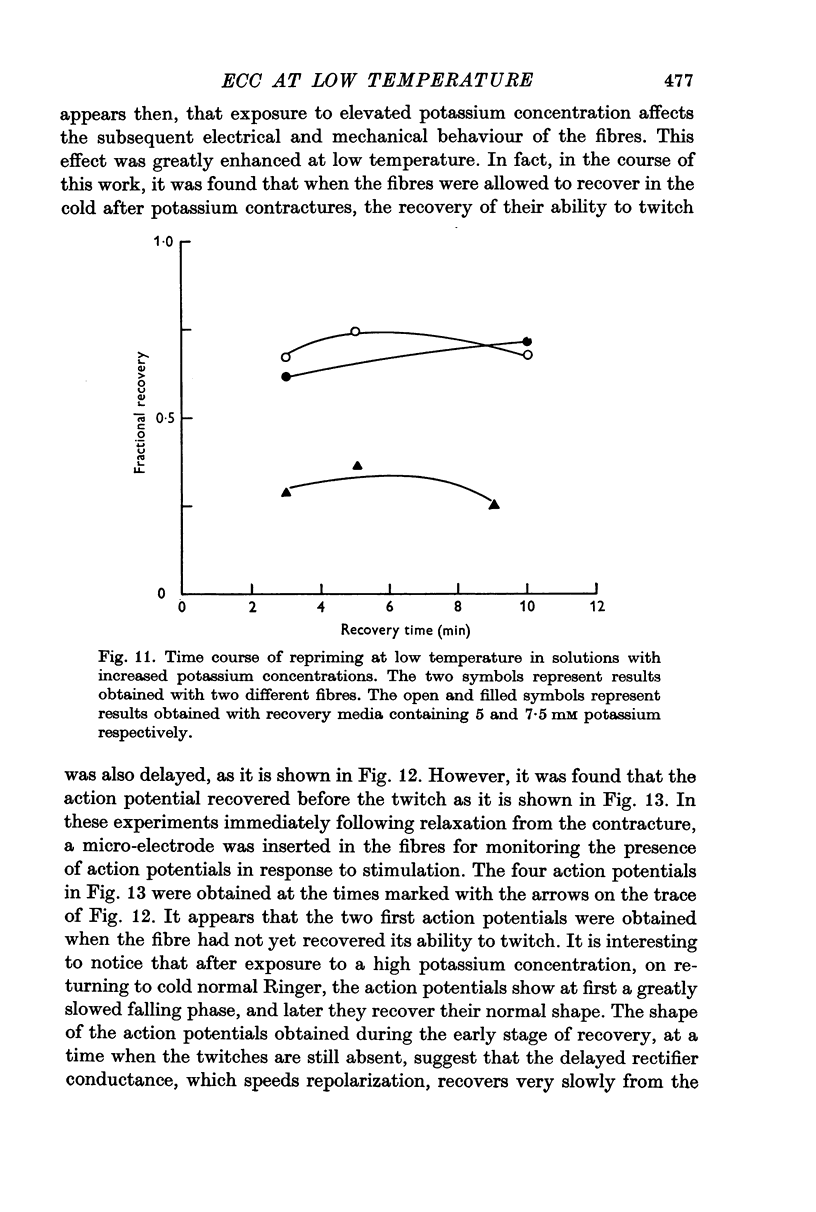
4. The repriming process, that is, the process by which the fibres recover their contractile ability following a potassium contracture, proceeds about six times slower at 3° C. This effect is not due to failure of the fibres to repolarize in the cold when transferred from a high potassium to a low potassium medium.
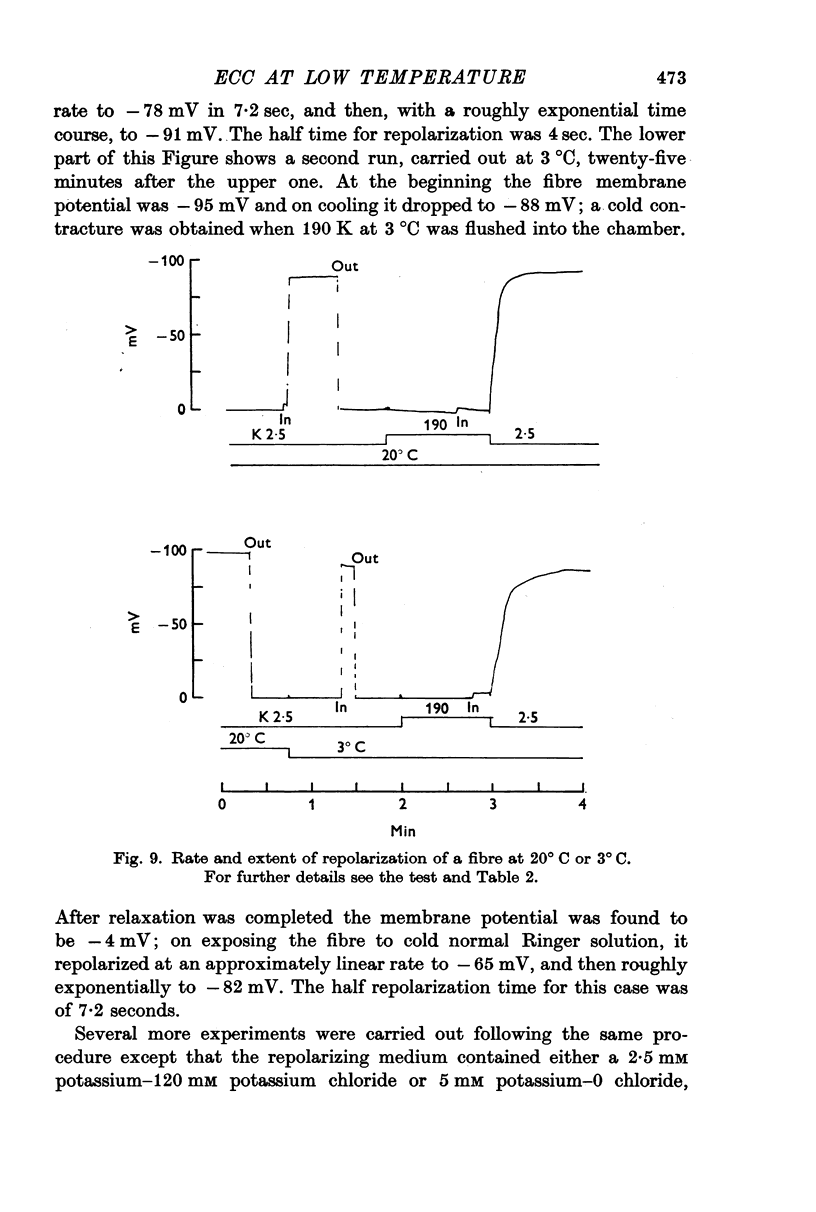
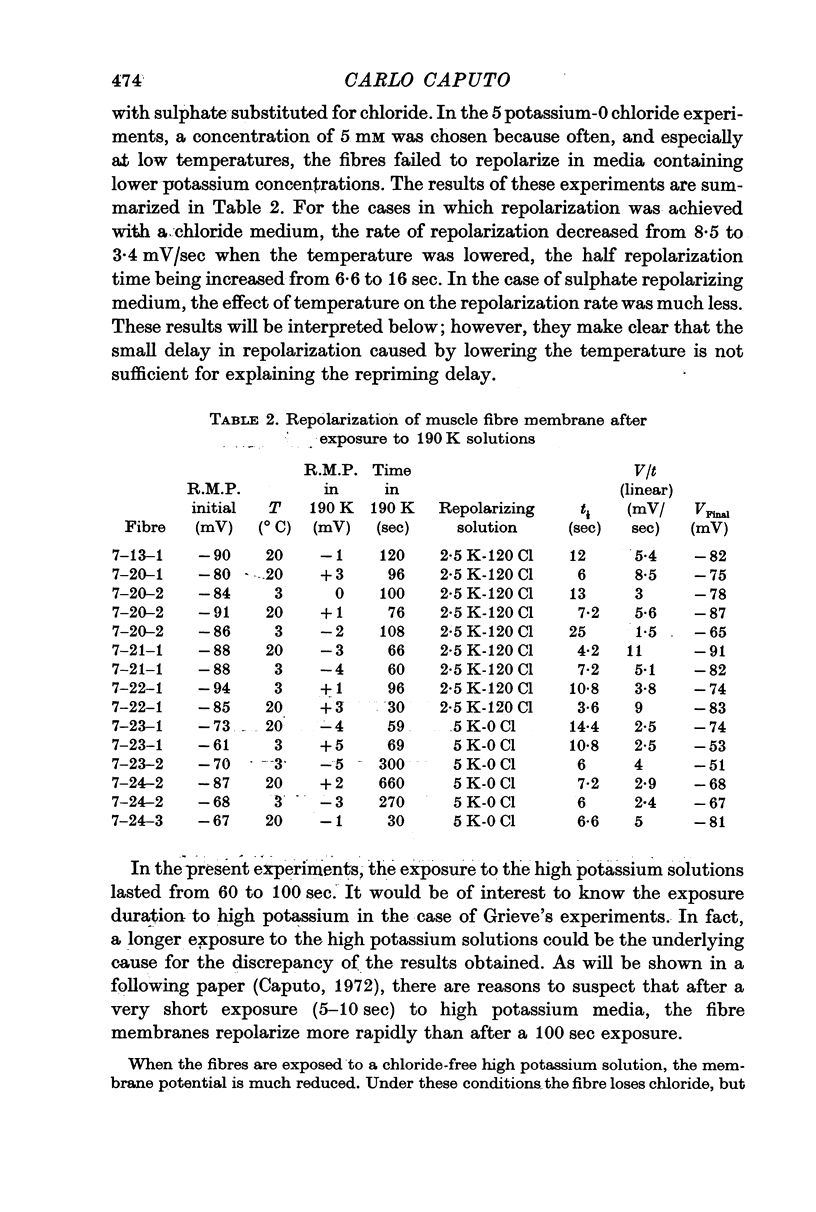
5. At low temperature repolarization occurs, even though it is somewhat slower. Following the solution change, from 190 mM potassium to a low potassium solution, the initial rate of repolarization is 8·5 mV/sec at 20° C, and 3·4 mV/sec at 3° C. This effect is not sufficient to account for the delay in the repriming process.
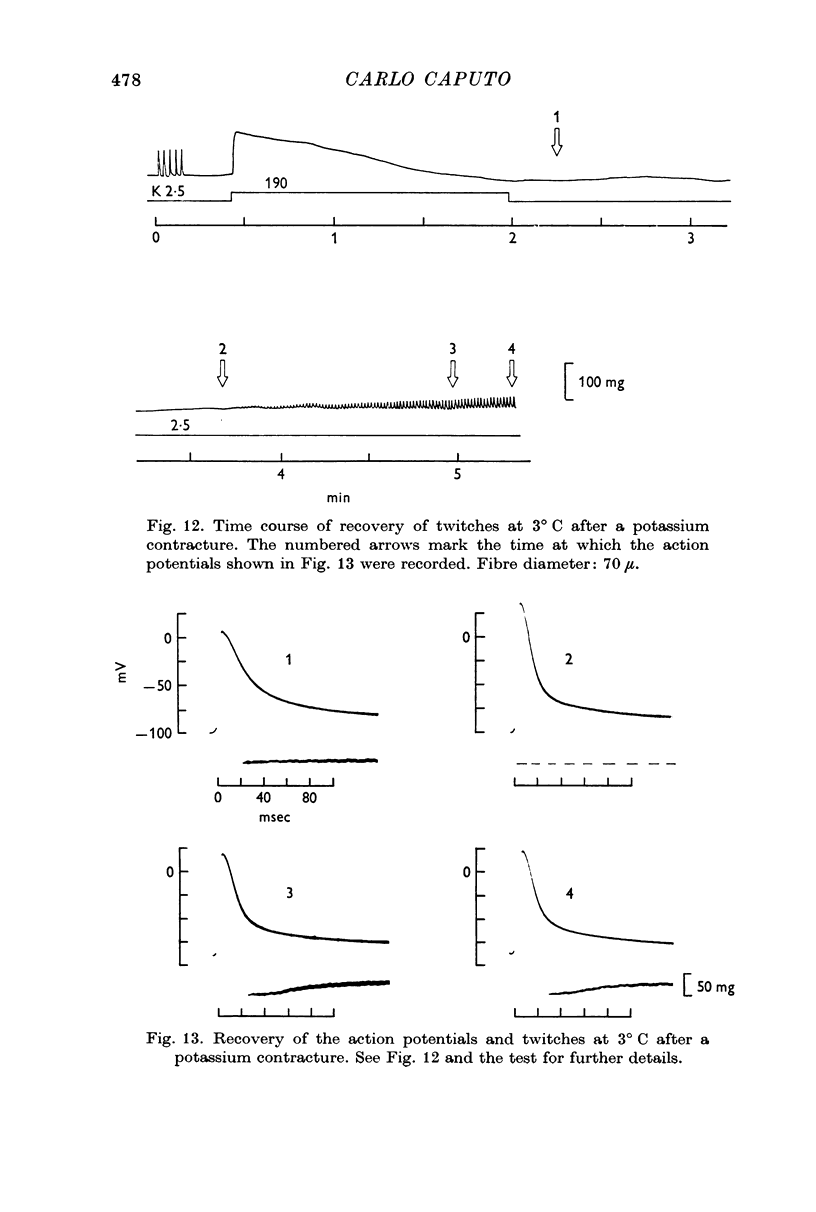
6. After a potassium contracture, recovery of the fibre's twitching ability at 3° C is also delayed. At a time when twitches have not yet been recovered, membrane potentials of -90 mV and almost normal action potentials can be recorded.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A., WILKIE D. R. The dynamics of the effect of potassium on frog's muscle. J Physiol. 1956 Dec 28;134(3):497–514. doi: 10.1113/jphysiol.1956.sp005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS B. A. THE RECOVERY OF CONTRACTILE ABILITY FOLLOWING A CONTRACTURE IN SKELETAL MUSCLE. J Gen Physiol. 1964 May;47:953–964. doi: 10.1085/jgp.47.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Caffeine- and potassium-induced contractures of frog striated muscle fibers in hypertonic solutions. J Gen Physiol. 1966 Sep;50(1):129–139. doi: 10.1085/jgp.50.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Gimenez M. Effects of external calcium deprivation on single muscle fibers. J Gen Physiol. 1967 Oct;50(9):2177–2195. doi: 10.1085/jgp.50.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. The time course of potassium contractures of single muscle fibres. J Physiol. 1972 Jun;223(2):483–505. doi: 10.1113/jphysiol.1972.sp009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., CARLSON F. D. POTASSIUM CONTRACTURES AND CREATINE PHOSPHATE BREAKDOWN IN FROG MUSCLE. Biochim Biophys Acta. 1964 Jul 29;88:213–215. doi: 10.1016/0926-6577(64)90170-6. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B., Lännergren J. The effect of calcium on the mechanical response of single twitch muscle fibres of Xenopus laevis. Acta Physiol Scand. 1967 Mar;69(3):242–254. doi: 10.1111/j.1748-1716.1967.tb03518.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Duffield J. C. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–1047. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. V., Edwards C. Some factors affecting the time course of the recovery of contracture ability following a potassium contracture in frog striated muscle. J Gen Physiol. 1965 Jul;48(6):975–983. [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Hodgkin A. L. Effect of diameter on the electrical constants of frog skeletal muscle fibres. Nature. 1970 Sep 5;227(5262):1053–1055. doi: 10.1038/2271053a0. [DOI] [PubMed] [Google Scholar]
- Winegrad S. The intracellular site of calcium activaton of contraction in frog skeletal muscle. J Gen Physiol. 1970 Jan;55(1):77–88. doi: 10.1085/jgp.55.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


