Abstract
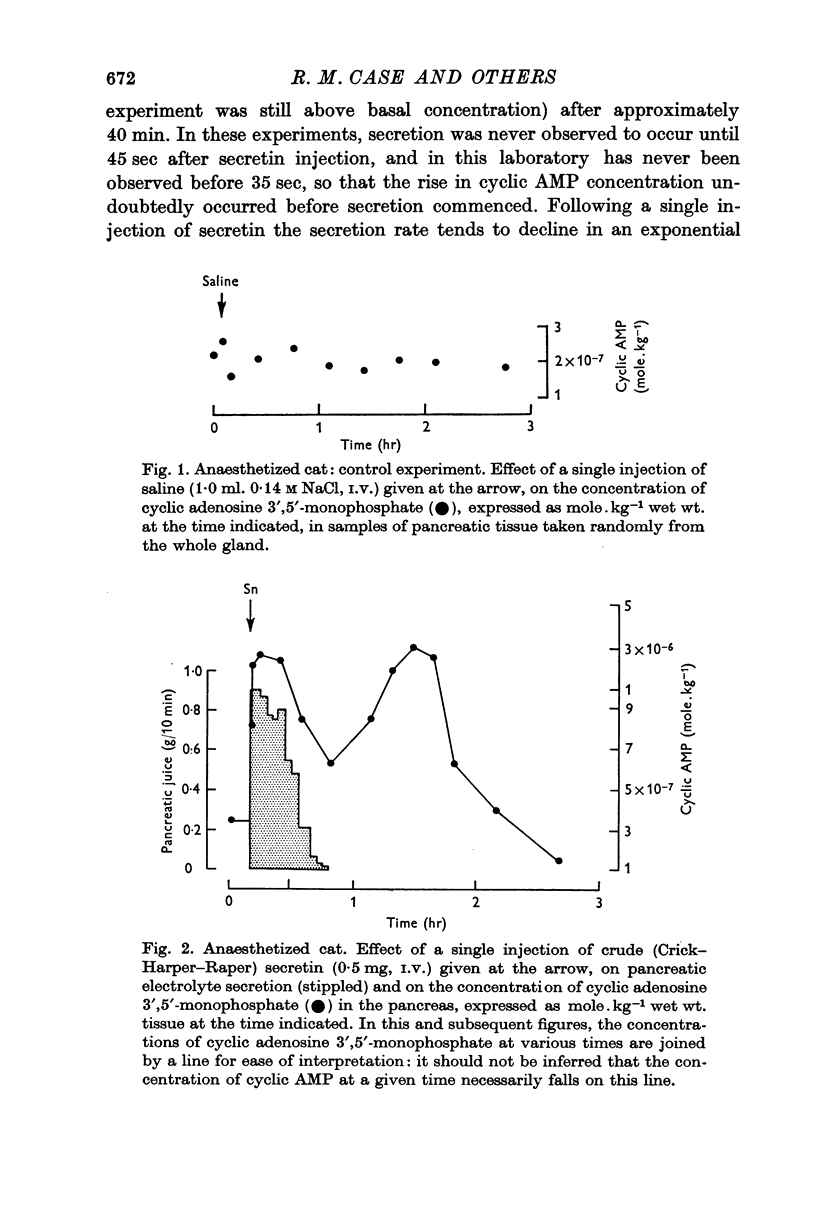
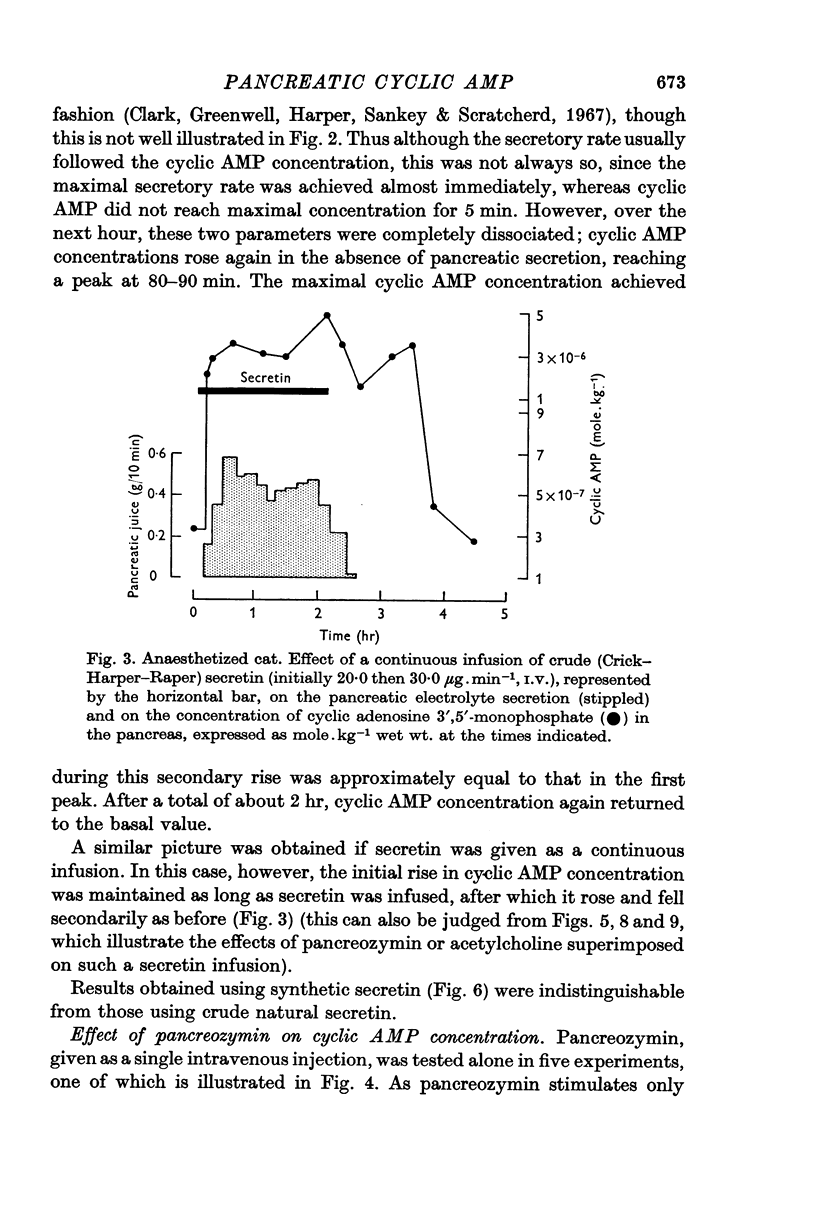
1. Following an I.V. injection of secretin into anaesthetized cats, the pancreatic cyclic AMP concentration rose within 30 sec and reached near-maximal values within 1 min. Pancreatic secretion began only after 45 sec. As secretion declined, the cyclic AMP concentration also fell. However, after 40 min, when secretion had ceased, the concentration again rose, reaching a maximum after about 80 min and returned to basal values within 140 min.
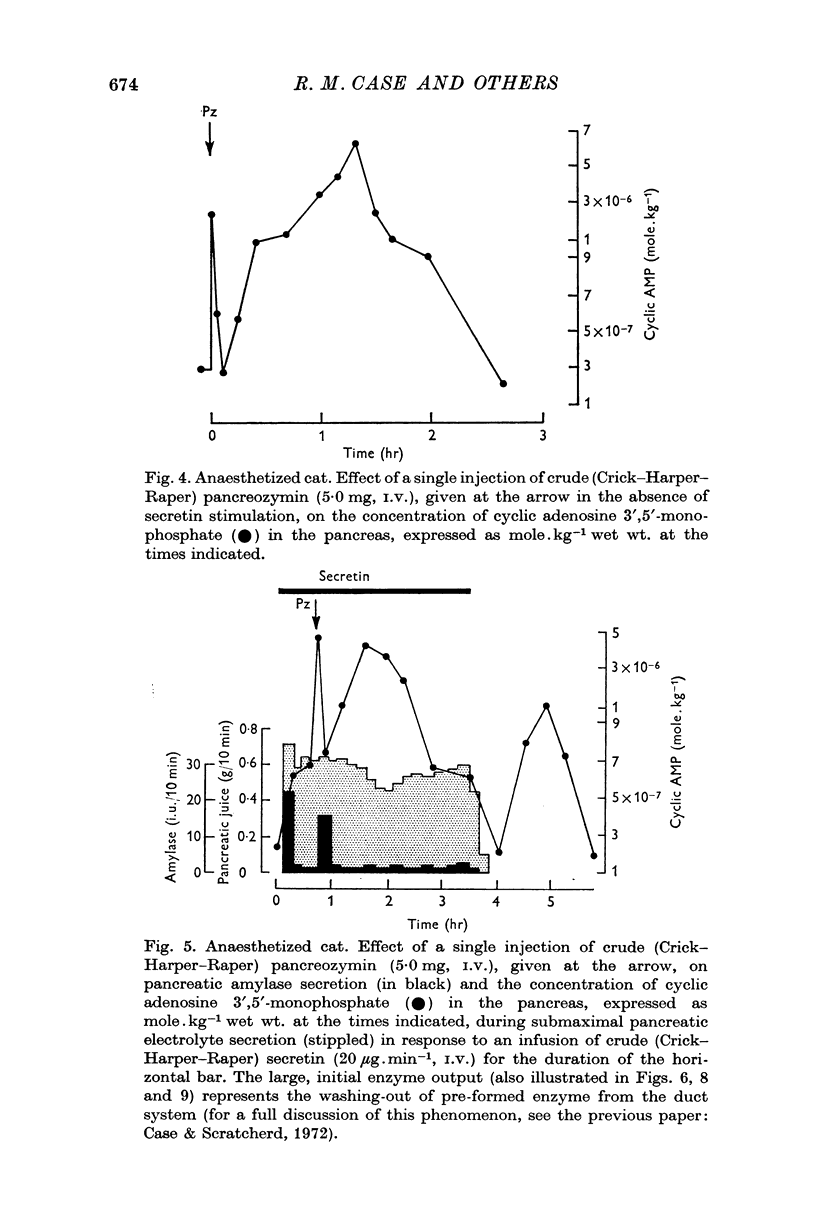
2. During secretin infusion the pattern of cyclic AMP changes was the same, except that the initial rise was maintained as long as secretin was infused.
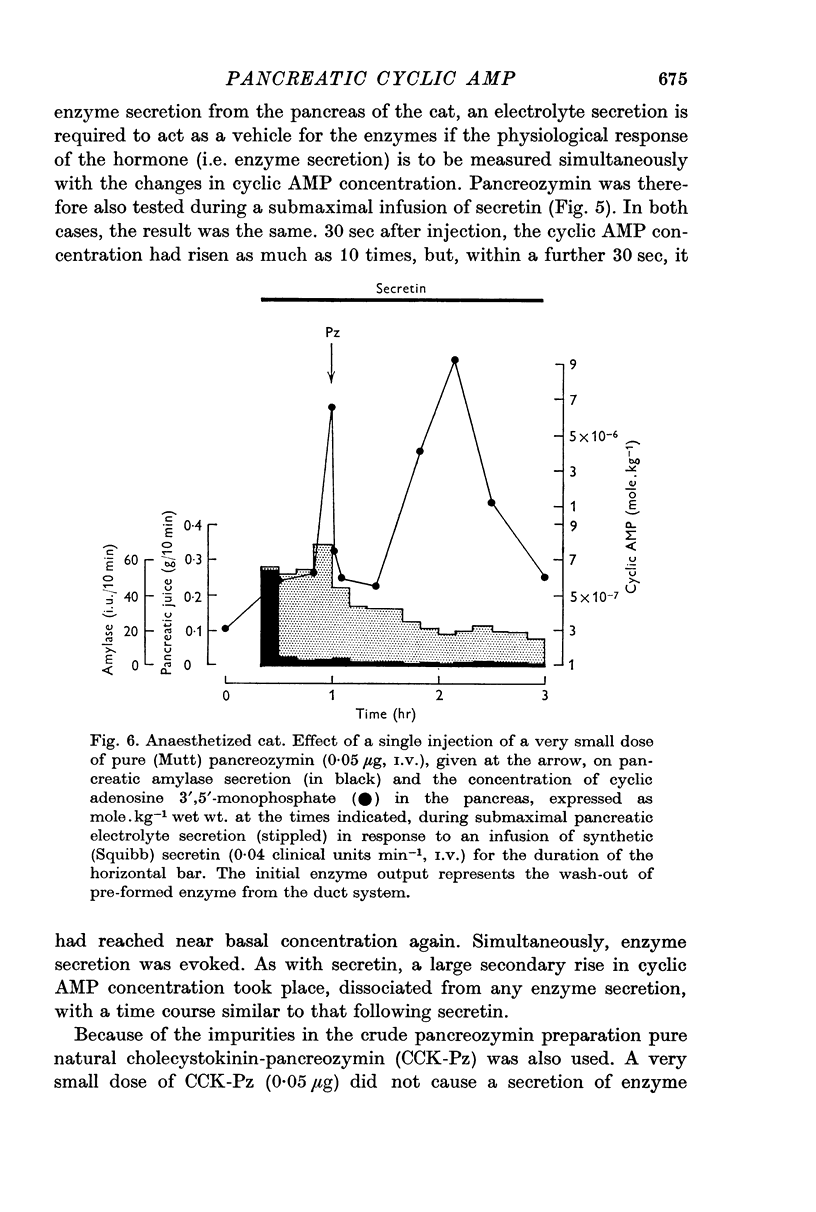
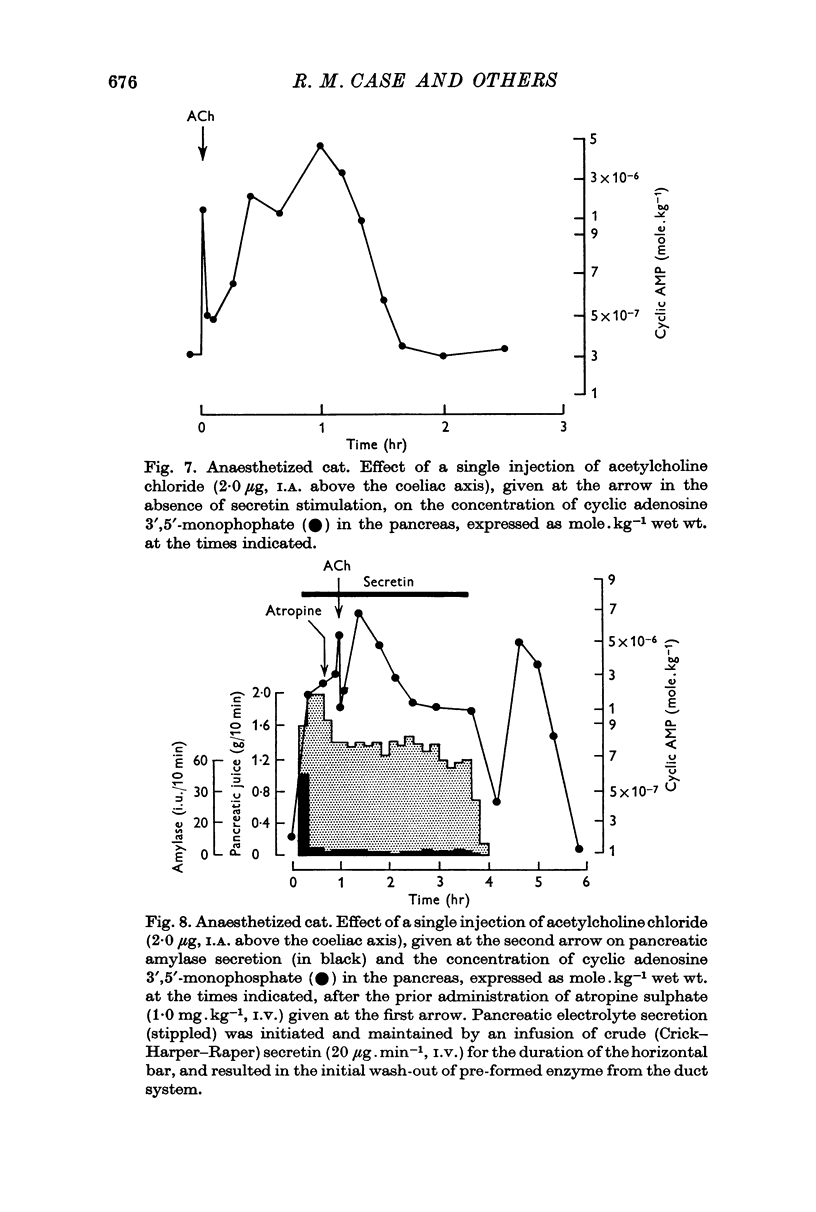
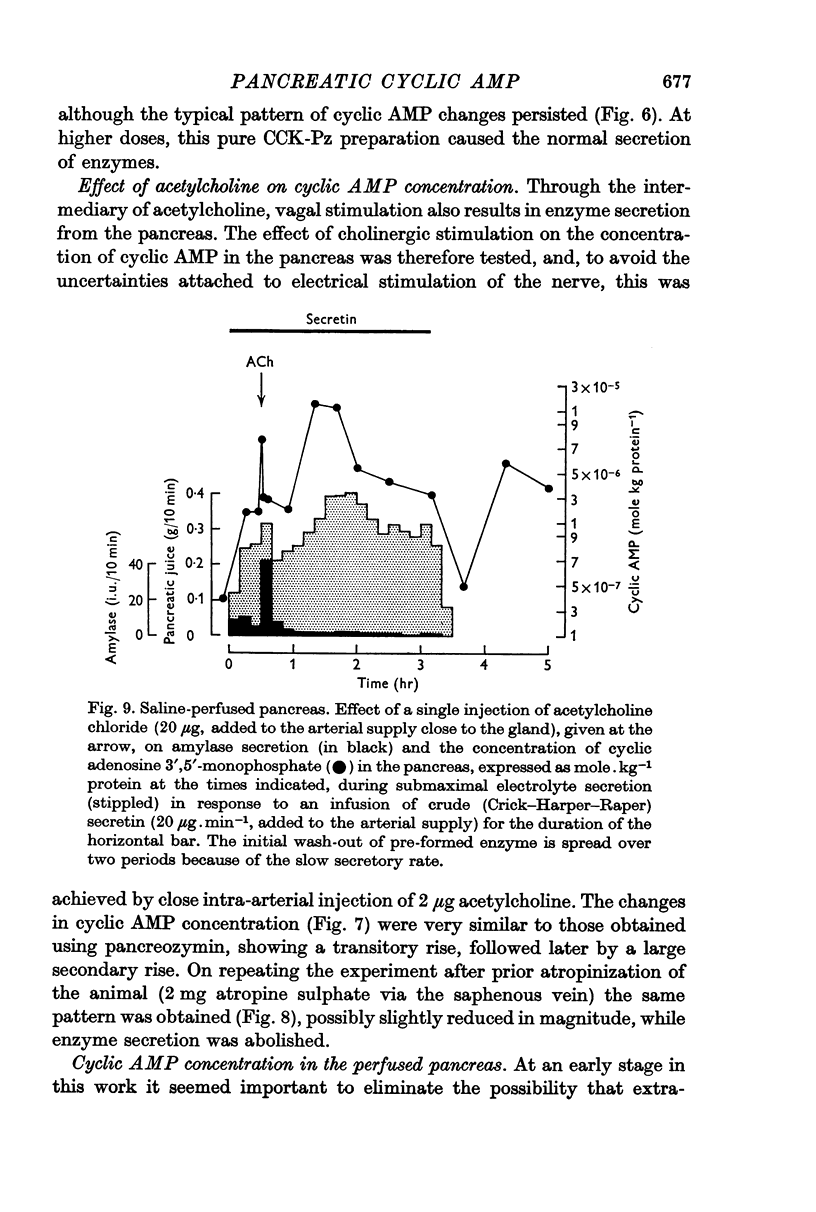
3. Following either pancreozymin or acetylcholine, alone or super-imposed on secretin stimulation, similar changes in cyclic AMP concentration were observed. However, the initial rise lasted only 30 sec, basal concentrations being approached within 1 min, and was accompanied by enzyme secretion. The concentration of cyclic AMP subsequently rose and fell again, in the absence of enzyme secretion, exactly as after secretin stimulation.
4. Similar observations were made using an isolated, saline-perfused preparation of the cat's pancreas.
5. By using very low doses of pancreozymin it was possible to observe the first rise in cyclic AMP concentration in the absence of enzyme secretion. Similarly atropine, while blocking enzyme secretion, did not affect the rise in cyclic AMP concentration after acetylcholine. The second increase in concentration was never associated with secretion (it may have been connected with the synthesis of exportable enzymes by the gland).
6. While these observations suggest that cyclic AMP may be involved in the response of the pancreas to secretin, pancreozymin and acetylcholine, no simple relation exists between cyclic AMP concentration and secretion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Butcher R. W., Carlson L. A. Effects of secretin on fat mobilizing lipolysis and cyclic AMP levels in rat adipose tissue. Acta Physiol Scand. 1970 Aug;79(4):559–563. doi: 10.1111/j.1748-1716.1970.tb04758.x. [DOI] [PubMed] [Google Scholar]
- CRICK J., HARPER A. A., RAPER H. S. On the preparation of secretin and pancreozymin. J Physiol. 1949 Dec;110(3-4):367–376. doi: 10.1113/jphysiol.1949.sp004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol. 1969 Apr;201(2):335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T. The actions of dibutyryl cyclic adenosine 3',5'-monophosphate and methyl xanthines on pancreatic exocrine secretion. J Physiol. 1972 Jun;223(3):649–667. doi: 10.1113/jphysiol.1972.sp009867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. W., Georg R. H., Bass A. D. Effect of glucagon, cyclic 3',5'-adenosine monophosphate and its dibutyryl derivative on amino acid uptake by the isolated perfused rat liver. Endocrinology. 1970 Aug;87(2):366–370. doi: 10.1210/endo-87-2-366. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Properties of cyclic 3',5'-nucleotide phosphodiesterase from rat brain. Biochemistry. 1967 Apr;6(4):1079–1087. doi: 10.1021/bi00856a017. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Greenwell J. R., Harper A. A., Sankey A. M., Scratcherd T. The electrical properties of resting and secreting pancreas. J Physiol. 1967 Apr;189(2):247–260. doi: 10.1113/jphysiol.1967.sp008166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I. The role of 3':5'-cyclic nucleotide phosphodiesterase in regulating tissue concentrations of adenosine 3':5'-cyclic mooophosphate. Biochem J. 1970 Dec;120(4):4P–5P. doi: 10.1042/bj1200004p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand R. J., Gross P. R. Independent smulation of secretion and protein snthesis in rat parotid gland. The influence of epinephrine and dibutyryl cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1969 Oct 25;244(20):5608–5615. [PubMed] [Google Scholar]
- Harris J. B., Nigon K., Alonso D. Adenosine-3',5'-monophosphate: intracellular mediator for methyl xanthine stimulation of gastric secretion. Gastroenterology. 1969 Oct;57(4):377–384. [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Johnson M., Sherratt H. S. A modified enzymic assay for adenosine 3':5'-cyclic monophosphate. Biochem J. 1970 Dec;120(4):7P–8P. doi: 10.1042/bj1200007p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Sherratt H. S., Case R. M., Scratcherd T. The effects of secretin, pancreozymin and acetylcholine on the concentration of adenosine 3':5'-cyclic monophosphate in cat pancreas. Biochem J. 1970 Dec;120(4):8P–9P. doi: 10.1042/bj1200008pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G. Inhibition of cyclic-3',5'-nucleotide-phosphodiesterase as a possible mode of action of papaverine and similarly acting drugs. Naunyn Schmiedebergs Arch Pharmakol. 1970;267(2):189–194. doi: 10.1007/BF00999402. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Loeb J. E., Blat C. Phosphorylation of some rat liver ribosomal proteins and its activation by cyclic AMP. FEBS Lett. 1970 Sep 24;10(2):105–108. doi: 10.1016/0014-5793(70)80427-6. [DOI] [PubMed] [Google Scholar]
- Menahan L. A., Hepp K. D., Wieland O. Liver 3':5'-nucleotide phosphodiesterase and its activity in rat livers perfused with insulin. Eur J Biochem. 1969 Apr;8(3):435–443. doi: 10.1111/j.1432-1033.1969.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Morisset J. A., Webster P. D. In vitro and in vivo effects of pancreozymin, urecholine, and cyclic AMP on rat pancreas. Am J Physiol. 1971 Jan;220(1):202–208. doi: 10.1152/ajplegacy.1971.220.1.202. [DOI] [PubMed] [Google Scholar]
- Okabayashi T., Ide M. Cyclic 3',5'-nucleotide phosphodiesterase of Serratia marcescens. Biochim Biophys Acta. 1970 Oct 14;220(1):116–123. doi: 10.1016/0005-2744(70)90235-4. [DOI] [PubMed] [Google Scholar]
- Phang J. M., Downing S. J., Weiss I. W. Cyclic AMP stimulation of amino acid uptake in bone and kidney. Biochim Biophys Acta. 1970 Sep 15;211(3):605–608. doi: 10.1016/0005-2736(70)90272-5. [DOI] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Biological significance of the prostaglandins. Recent Prog Horm Res. 1970;26:139–187. doi: 10.1016/b978-0-12-571126-5.50008-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Christophe J. Secretion of hydrolases by perfused fragments of rat pancreas: effect of calcium. Am J Physiol. 1971 Apr;220(4):911–917. doi: 10.1152/ajplegacy.1971.220.4.911. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L. Adenyl cyclase in fat cells. 3. Stimulation by secretin and the effects of trypsin on the receptors for lipolytic hormones. J Biol Chem. 1970 Feb 25;245(4):718–722. [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- Schulz I., Yamagata A., Weske M. Micropuncture studies on the pancreas of the rabbit. Pflugers Arch. 1969;308(3):277–290. doi: 10.1007/BF00586559. [DOI] [PubMed] [Google Scholar]
- Wormsley K. G. Responses to secretin and pancreozymin in man. J Physiol. 1969 Jul;203(1):53P–54P. [PubMed] [Google Scholar]


