Abstract
1. The effects of anaesthetics (pentobarbitone, hexobarbitone, halothane, urethane, chloralose, chloral hydrate and ethanol) on the extracellular field potentials of the olfactory bulb produced by lateral olfactory tract stimulation were analysed.
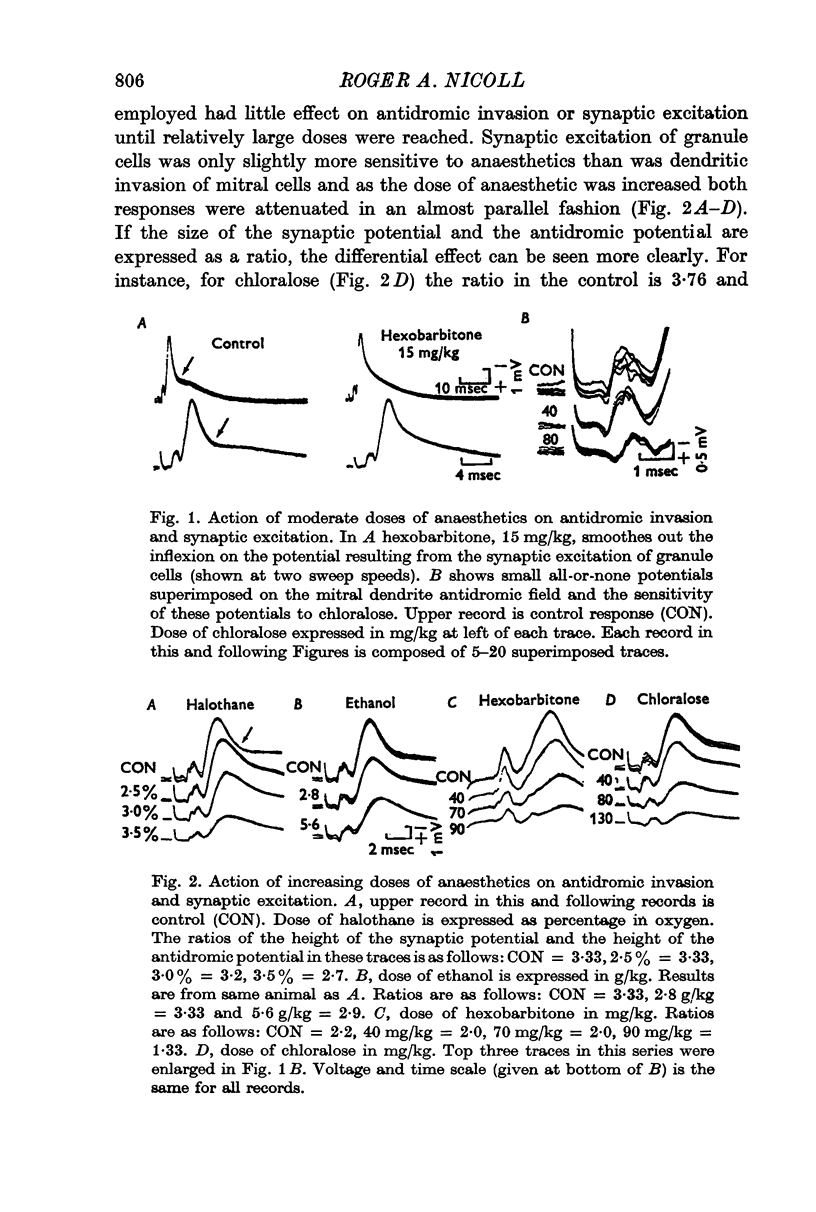
2. Relatively large doses of all the anaesthetics (e.g. pentobarbitone, 40-70 mg/kg) depressed the synaptic excitation of granule cells.
3. The antidromic invasion of mitral cell dendrites was only slightly less sensitive to the anaesthetics than was the synaptic excitation of granule cells.
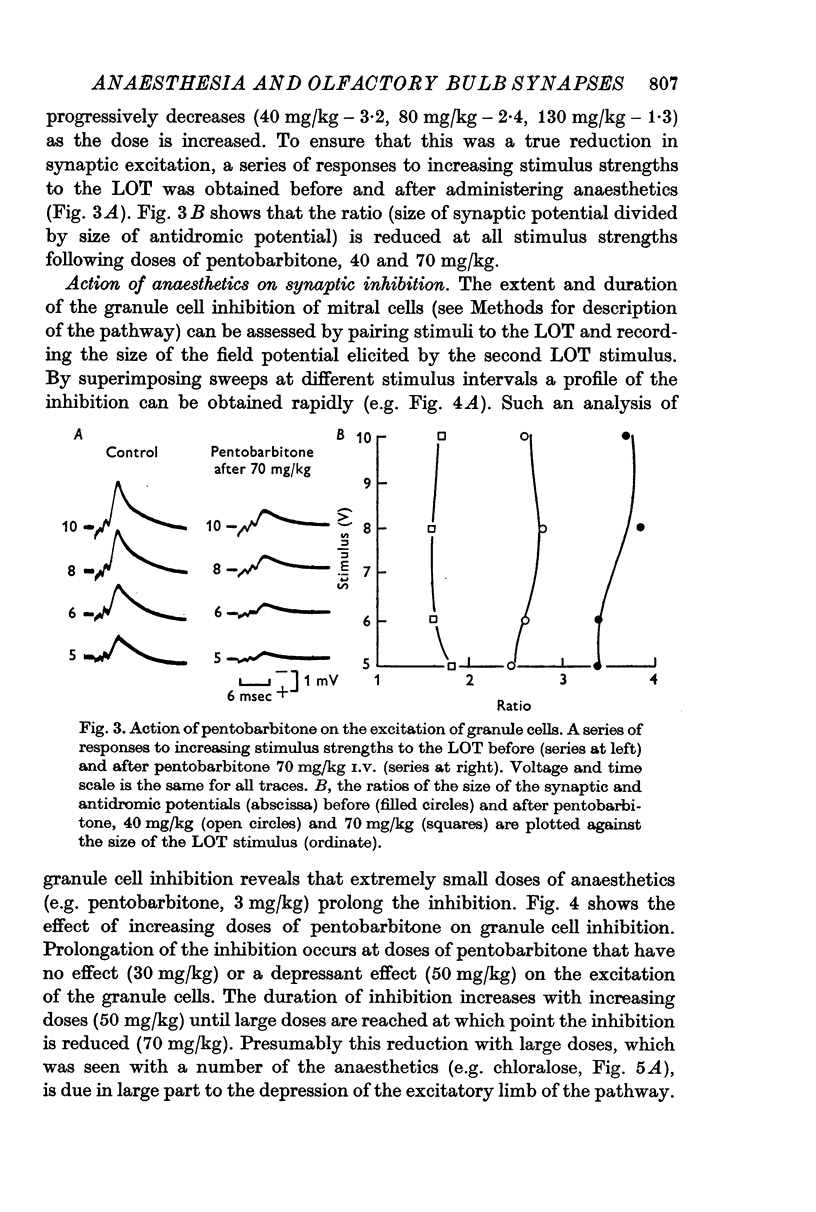
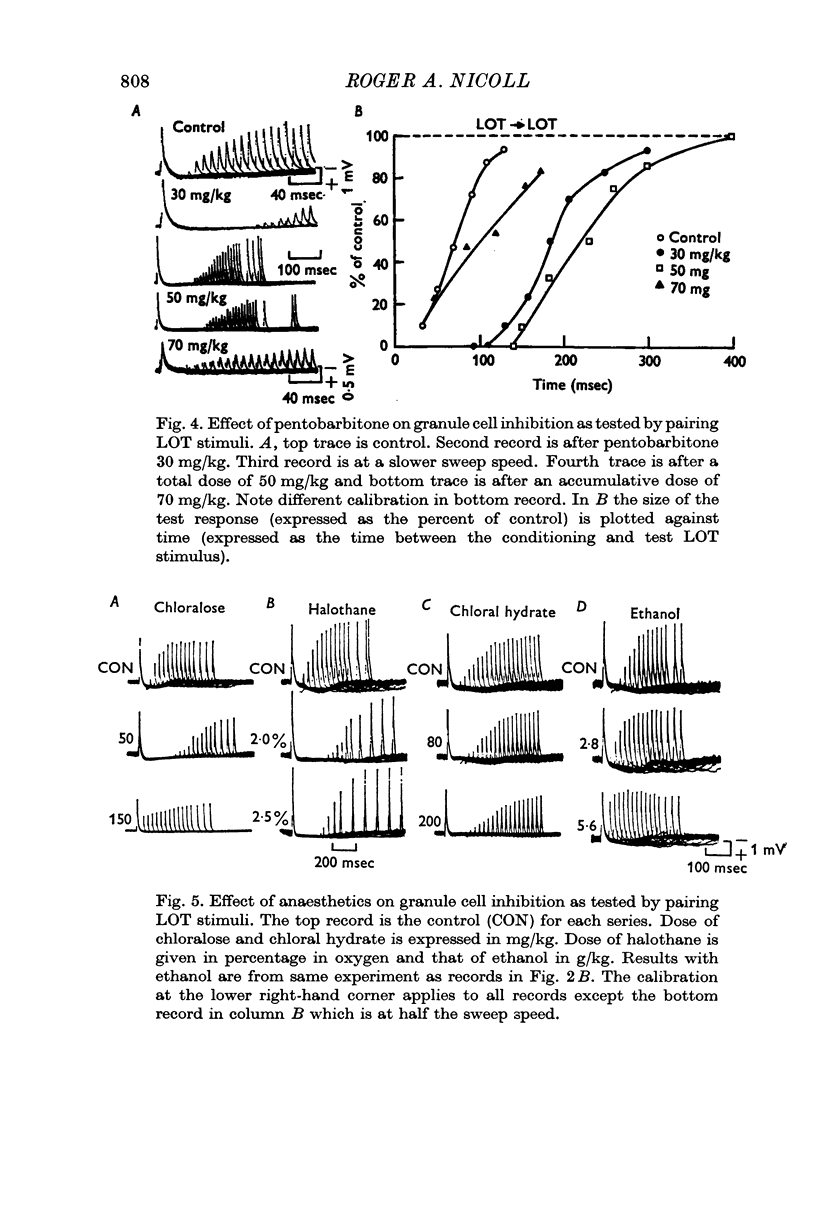
4. A wide dose range of anaesthetics (e.g. pentobarbitone, 3-60 mg/kg) prolonged the granule cell post-synaptic inhibition of mitral cells. All the anaesthetics, except ethanol, prolonged the inhibition.
5. The action of anaesthetics on post-synaptic inhibition was due to a specific effect on the inhibitory synapses.
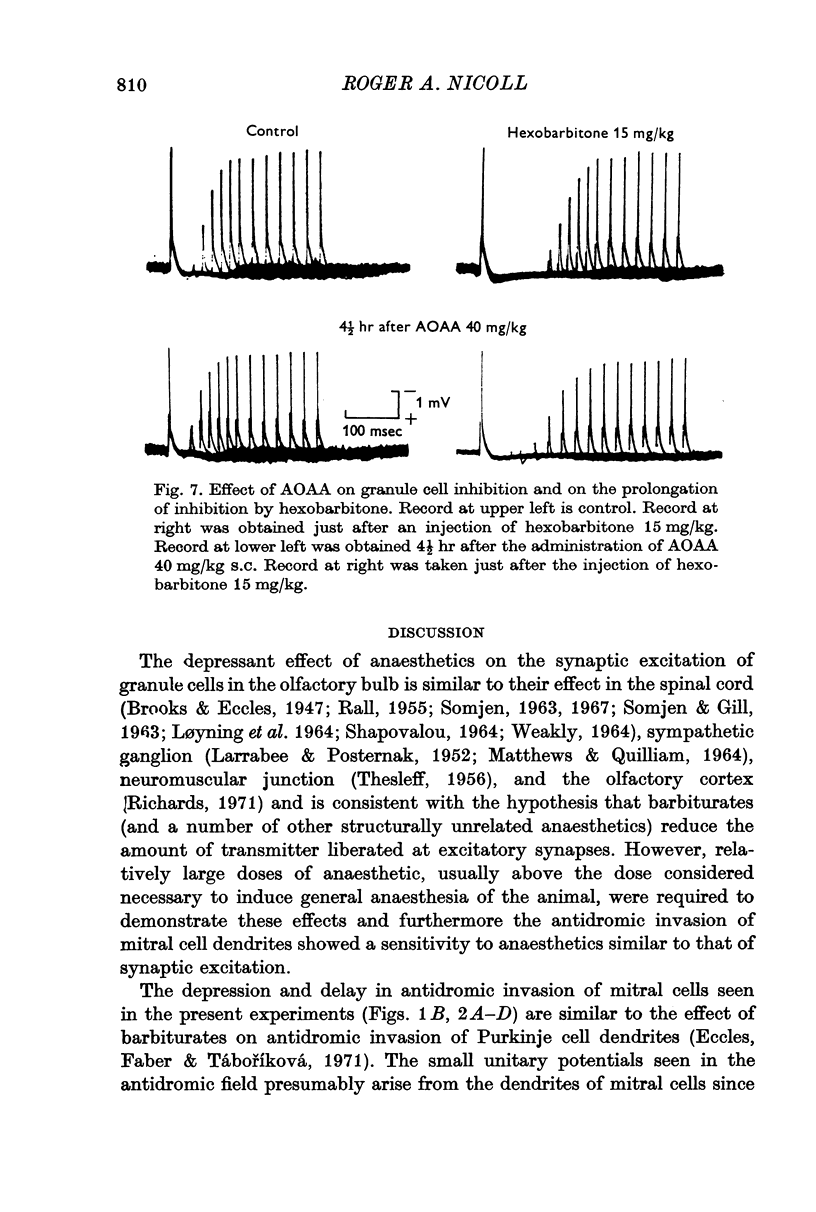
6. Amino-oxyacetic acid, an inhibitor of GABA catabolism, had little effect on the synaptic inhibition or on the ability of hexobarbital to prolong the inhibition. This suggests that the prolongation seen with anaesthetics is not a result of interfering with GABA catabolism.
7. The present results are compared with results obtained with anaesthetics in other areas of the nervous system and it is proposed that prolongation of `gaba-ergic' inhibition might contribute to an agent's ability to produce general anaesthesia.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES K. H. DER FEINBAU DES BULBUS OFACTORIUS DER RATTE UNTER BESONDERER BERUECKSICHTIGUNG DER SYNAPTISCHEN VERBINDUNGEN. Z Zellforsch Mikrosk Anat. 1965 Feb 9;65:530–561. [PubMed] [Google Scholar]
- Bloedel J. R., Roberts W. J. Functional relationship among neurons of the cerebellar cortex in the absence of anesthesia. J Neurophysiol. 1969 Jan;32(1):75–84. doi: 10.1152/jn.1969.32.1.75. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Felix D. The effect of bicuculline upon synaptic inhibition in the cerebral and cerebellar corticles of the cat. Brain Res. 1971 Nov;34(2):301–321. doi: 10.1016/0006-8993(71)90283-6. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Táboríková H. The action of a parallel fiber volley on the antidromic invasion of Purkyne cells of cat cerebellum. Brain Res. 1971 Jan 22;25(2):335–356. doi: 10.1016/0006-8993(71)90442-2. [DOI] [PubMed] [Google Scholar]
- Felix D., McLennan H. The effect of bicuculline on the inhibition of mitral cells of the olfactory bulb. Brain Res. 1971 Feb 5;25(3):661–664. doi: 10.1016/0006-8993(71)90472-0. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- Larson M. D., Major M. A. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970 Jul 14;21(2):309–311. doi: 10.1016/0006-8993(70)90377-x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS E. K., QUILLIAM J. P. EFFECTS OF CENTRAL DEPRESSANT DRUGS UPON ACETYLCHOLINE RELEASE. Br J Pharmacol Chemother. 1964 Apr;22:415–440. doi: 10.1111/j.1476-5381.1964.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H. The pharmacology of inhibition of mitral cells in the olfactory bulb. Brain Res. 1971 Jun 18;29(2):177–184. doi: 10.1016/0006-8993(71)90026-6. [DOI] [PubMed] [Google Scholar]
- Miyahara J. T., Esplin D. W., Zablocka B. Differential effects of depressant drugs on presynaptic inhibition. J Pharmacol Exp Ther. 1966 Oct;154(1):119–127. [PubMed] [Google Scholar]
- Nicoll R. A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 1969 Jun;14(1):157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Olfactory nerves and their excitatory action in the olfactory bulb. Exp Brain Res. 1972;14(2):185–197. doi: 10.1007/BF00234798. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pharmacological evidence for GABA as the transmitter in granule cell inhibition in the olfactory bulb. Brain Res. 1971 Dec 10;35(1):137–149. doi: 10.1016/0006-8993(71)90600-7. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. The synaptology of the granule cells of the olfactory bulb. J Cell Sci. 1970 Jul;7(1):125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Richards C. D. The selective depression of evoked cortical EPSPs by pentobarbitone. J Physiol. 1971;217 (Suppl):41P–43P. [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- SOMJEN G. G. Effects of ether and thiopental on spinal presynaptic terminals. J Pharmacol Exp Ther. 1963 Jun;140:396–402. [PubMed] [Google Scholar]
- SOMJEN G. G., GILL M. The mechanism of the blockade of synaptic transmission in the mammalian spinal cord by diethyl ether and by thiopental. J Pharmacol Exp Ther. 1963 Apr;140:19–30. [PubMed] [Google Scholar]
- Somjen G. Effects of anesthetics on spinal cord of mammals. Anesthesiology. 1967 Jan-Feb;28(1):135–143. doi: 10.1097/00000542-196701000-00015. [DOI] [PubMed] [Google Scholar]
- WALLACH D. P. Studies on the GABA pathway. I. The inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase in vitro and in vivo by U-7524 (amino-oxyacetic acid). Biochem Pharmacol. 1961 Feb;5:323–331. doi: 10.1016/0006-2952(61)90023-5. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N., Esplin D. W., Zablocka B. Criteria for assessing effects of drugs on postsynaptic inhibition. Arch Int Pharmacodyn Ther. 1968 Feb;171(2):385–393. [PubMed] [Google Scholar]
- Westecker M. E. Reciprocal activation of two evoked potential components in the olfactory bulb. Pflugers Arch. 1971;324(4):297–310. doi: 10.1007/BF00592458. [DOI] [PubMed] [Google Scholar]


