Abstract
1. The superior colliculus has been studied in Siamese and normal cats by recording the responses of single tectal units to visual stimuli.
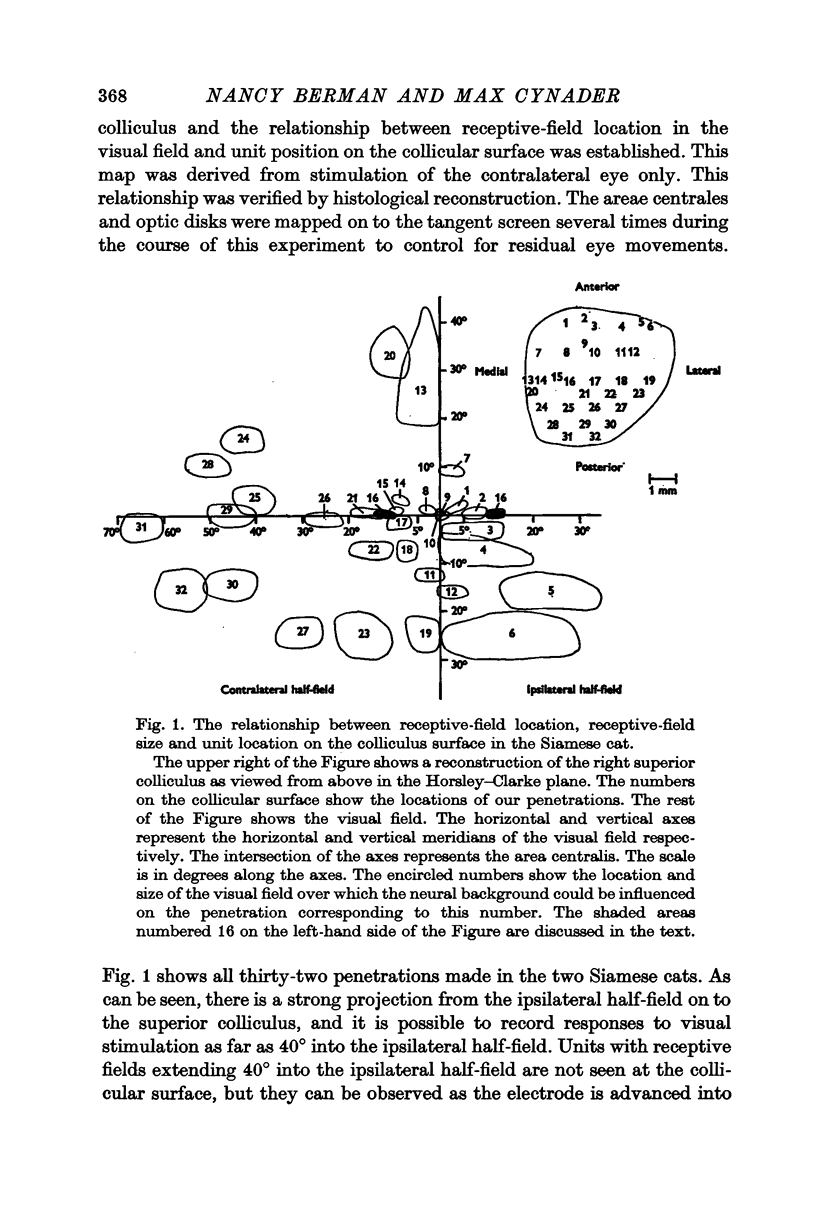
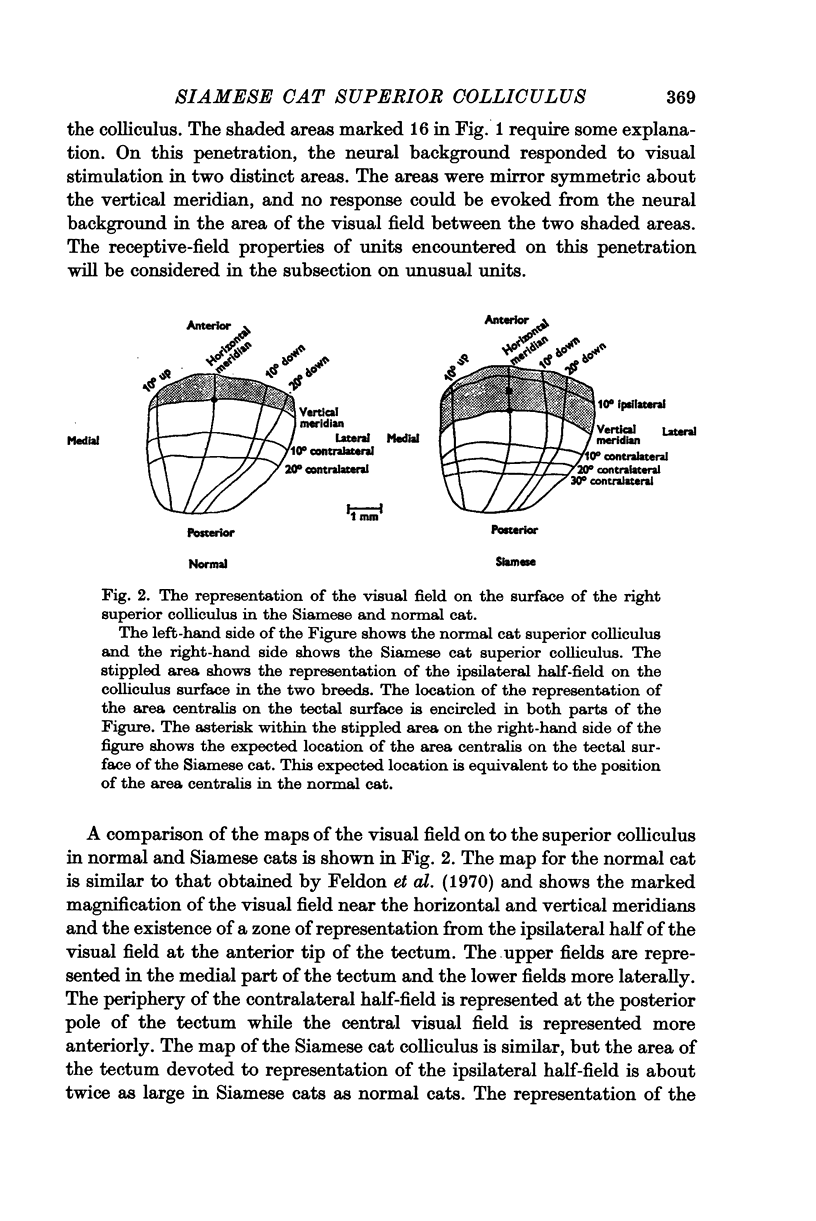
2. The retinotopic organization of the superior colliculus has been compared in the two breeds. In the normal cat, the contralateral half-field is represented in the central and caudal part of the colliculus, and a vertical strip of the ipsilateral half-field, 15-20° wide, is represented at the anterior tip. The Siamese cat superior colliculus receives an abnormally large projection from the ipsilateral half-field so that units with visual receptive fields which extend as far as 40° into the ipsilateral half-field can be found. The area of the tectal surface devoted to the representation of the ipsilateral half-field is about twice as large in Siamese cats as in normal cats. The enhanced representation of the ipsilateral half-field in Siamese cats is reflected in a displacement of the vertical meridian and the area centralis on the tectal surface.
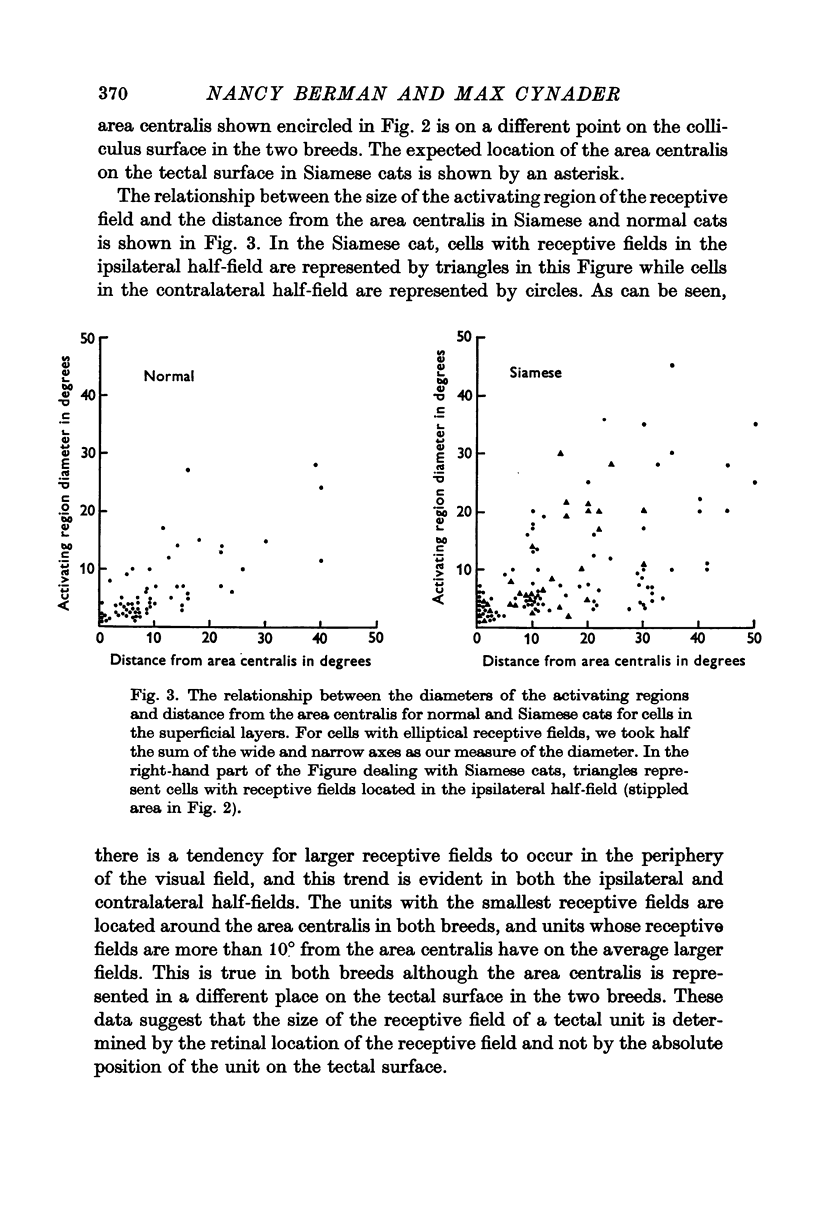
3. The area centralis in the Siamese cat is located at about the same point on the tectal surface as would be occupied by a point in the visual field about 6-7° contralateral to the area centralis in the normal cat. The smallest receptive fields in both breeds are located near the area centralis. The size of the receptive field for a tectal unit seems to be determined by the retinal location of the receptive field and not by the absolute position of the unit on the tectal surface.
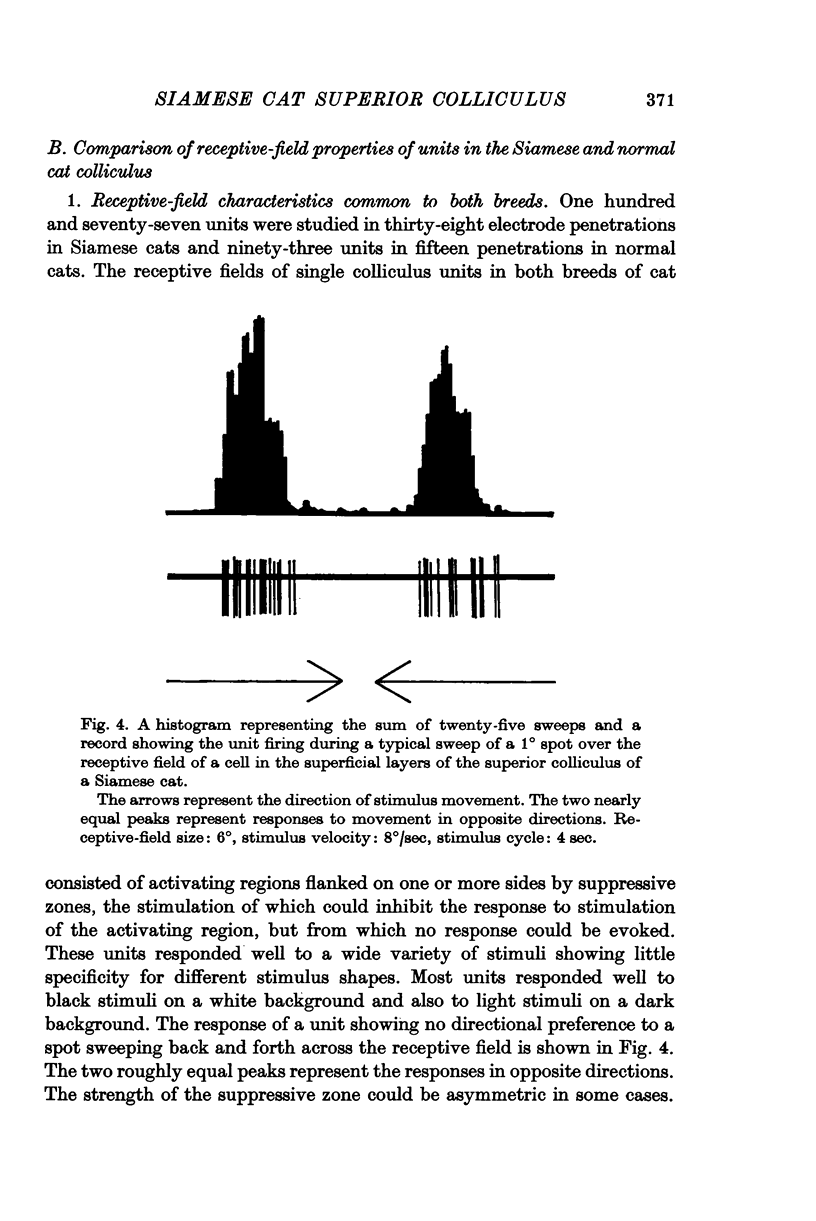
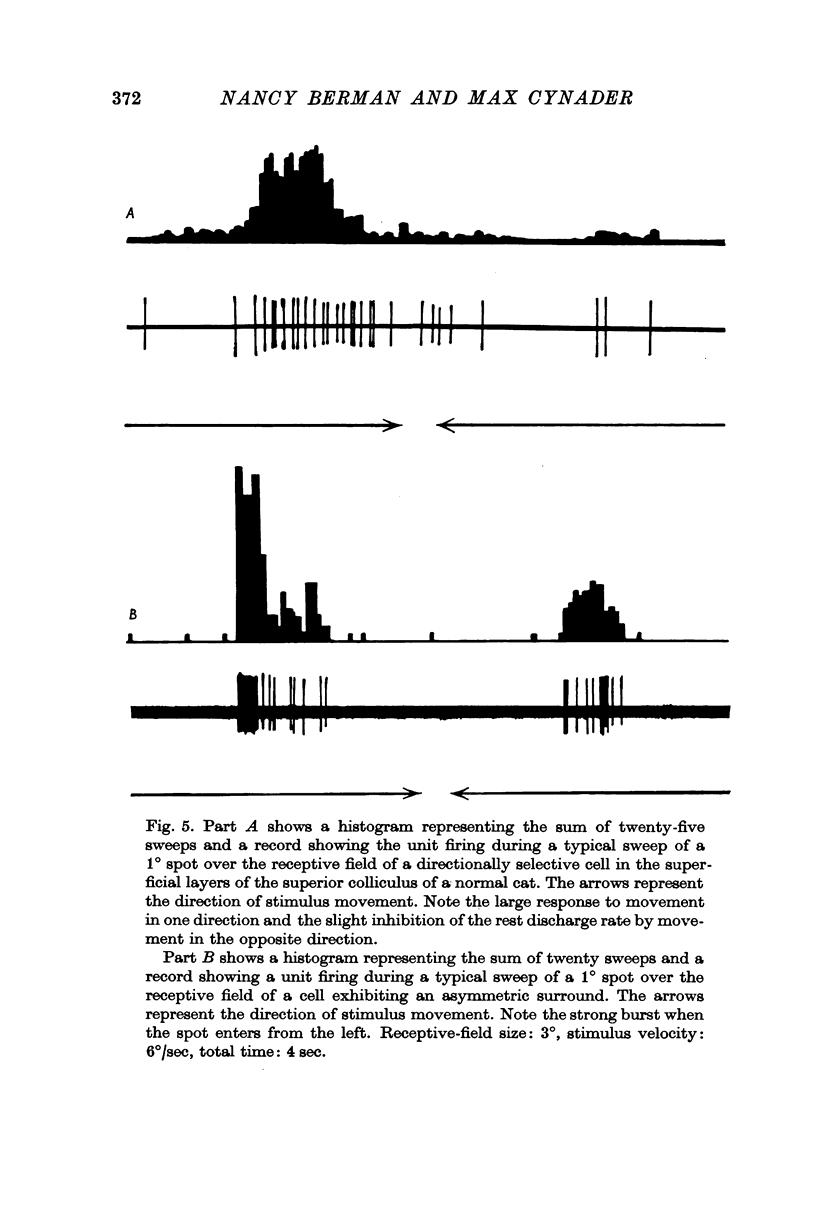
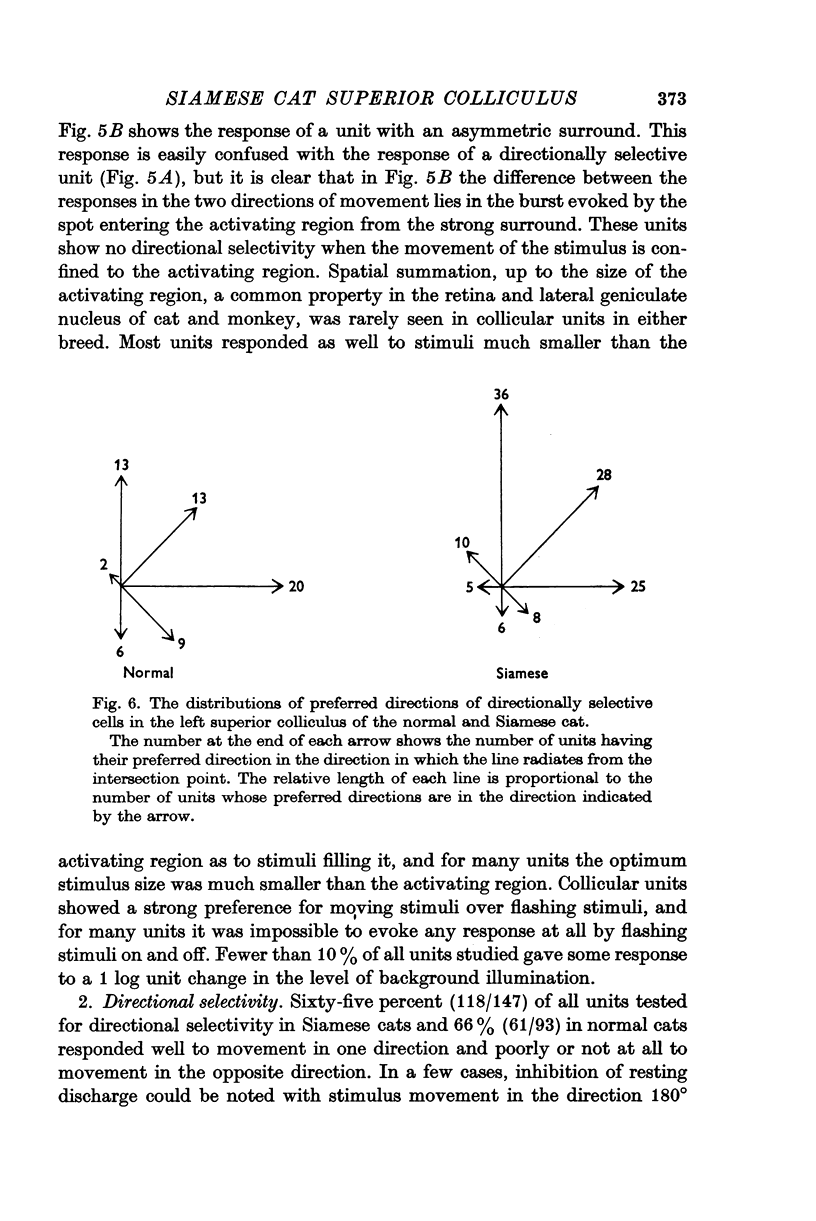
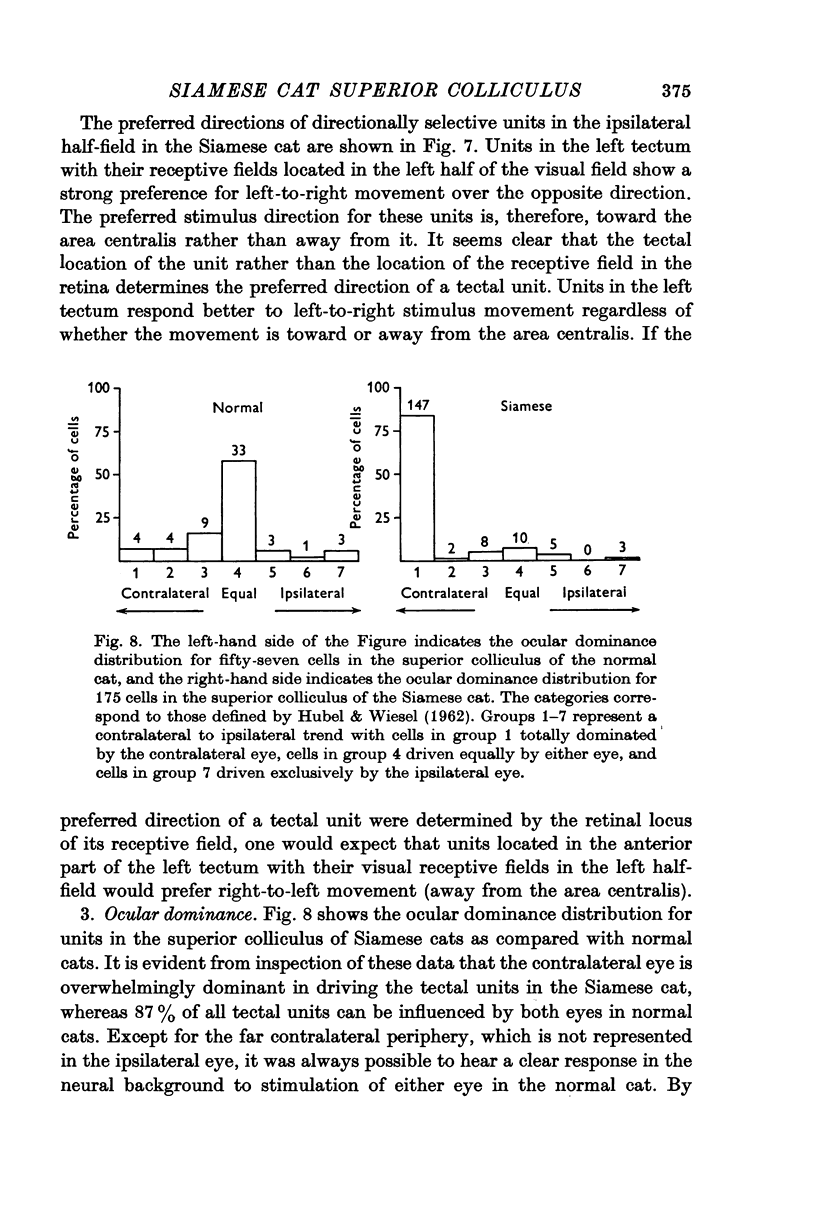
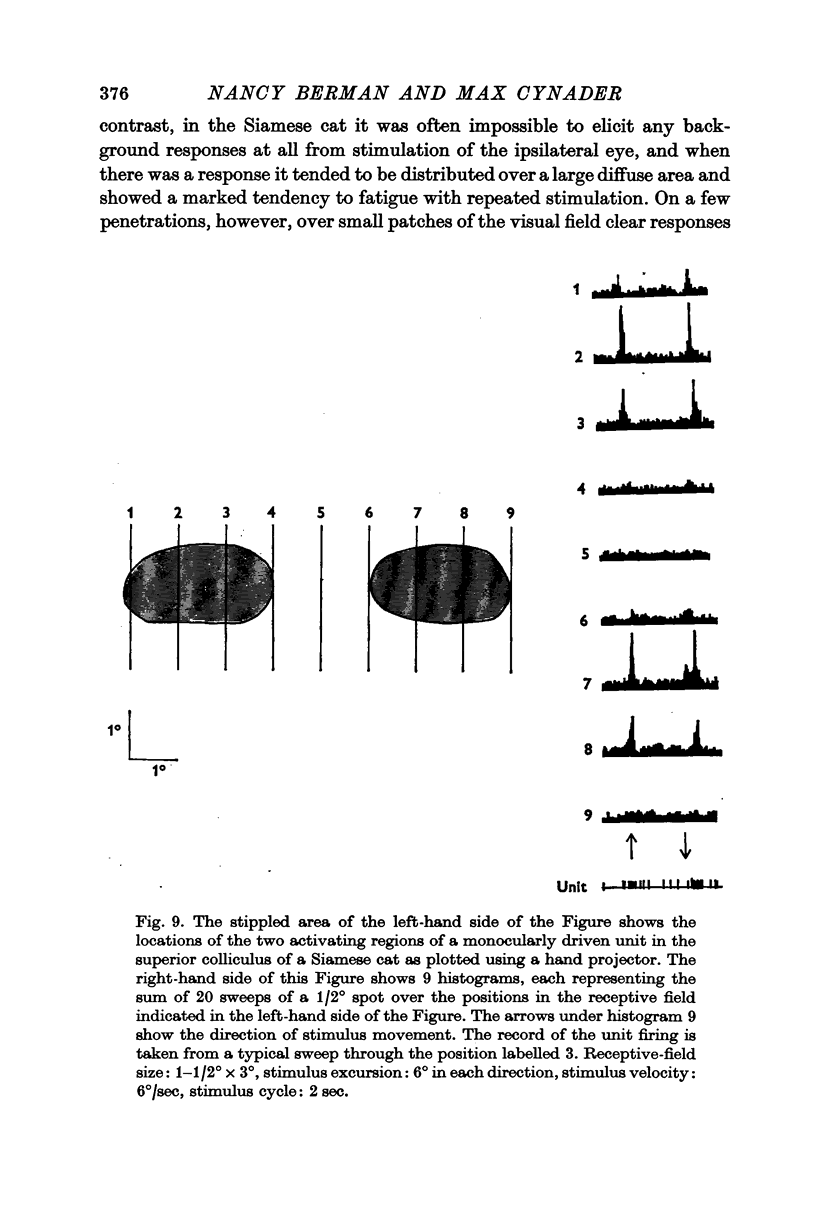
4. The receptive-field characteristics of tectal units show many similarities in the two breeds. The receptive fields of individual units consist of activating regions flanked by suppressive surrounds. Units respond well to stimuli of different shapes and orientation provided they are moving. The optimum stimulus for a given unit can be much smaller than the size of the activating region. About two thirds of the units studied in both breeds show directional selectivity. Most of the units studied in normal cats can be activated by stimulation of either eye, while in Siamese cats, 80% of the units studied can be driven only by the contralateral eye. A few monocularly driven units with two separated receptive fields have been observed in Siamese cats.
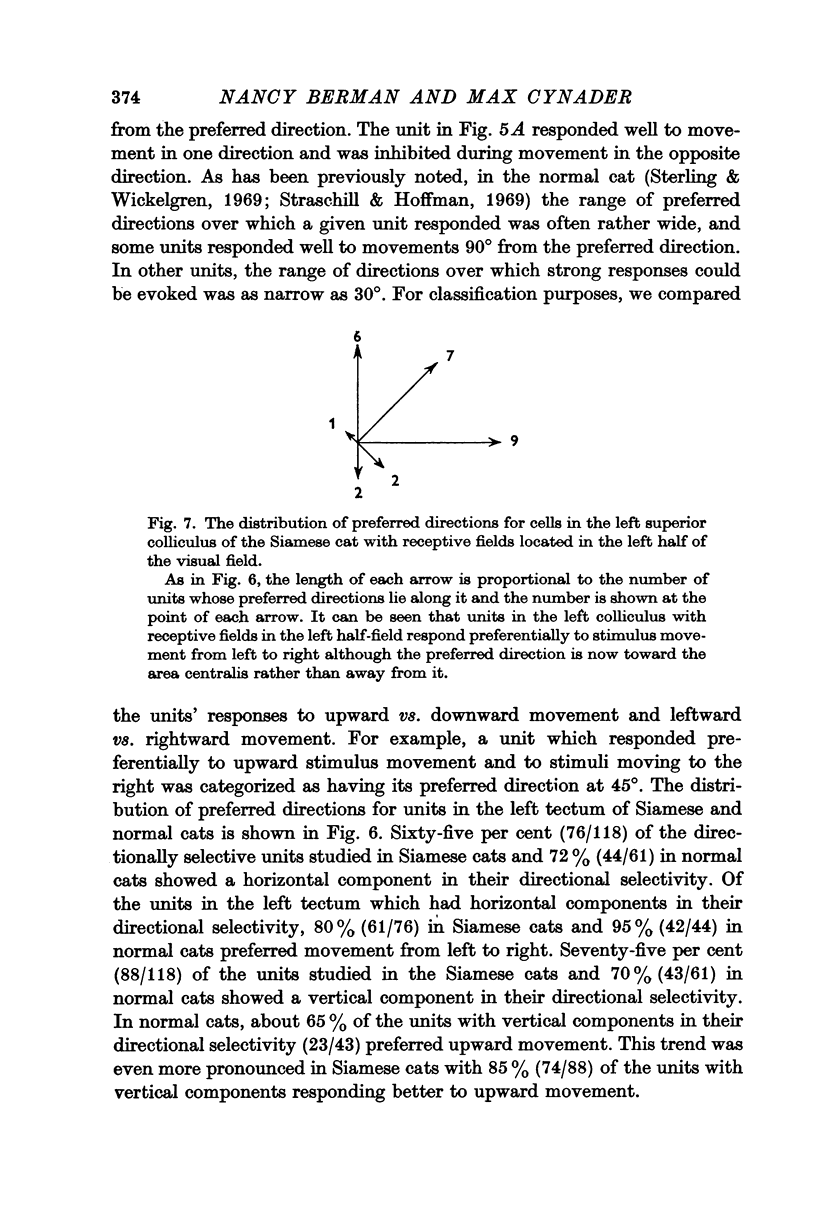
5. In the left tectum of both breeds, units respond well to left-to-right stimulus movement. The reverse situation obtains in the right tectum. In Siamese cats, units located at the anterior tip of the tectum with their receptive fields located in the visual half-field ipsilateral to the tectum under study respond better to stimulus movement toward the area centralis than away from it. The preferred direction for a tectal unit seems to be determined by its tectal location rather than by the location of its receptive field in the retina.
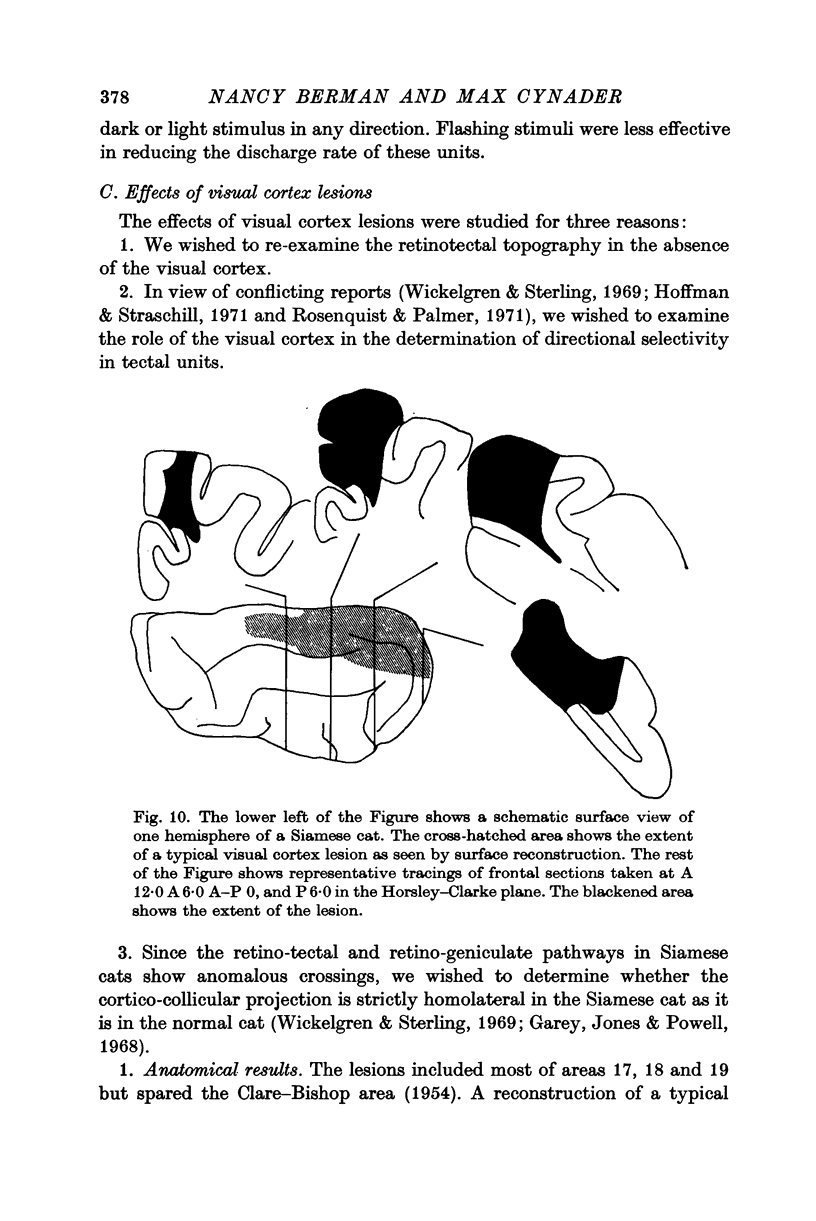
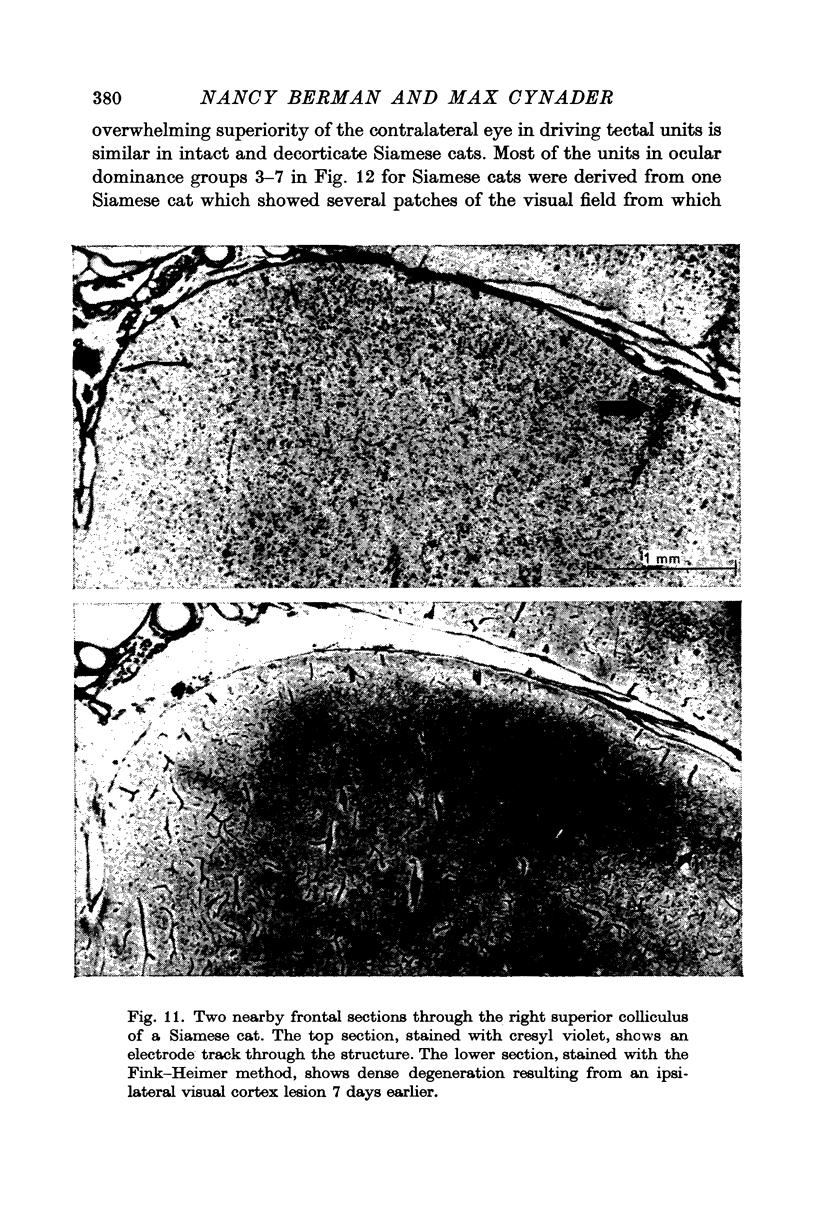
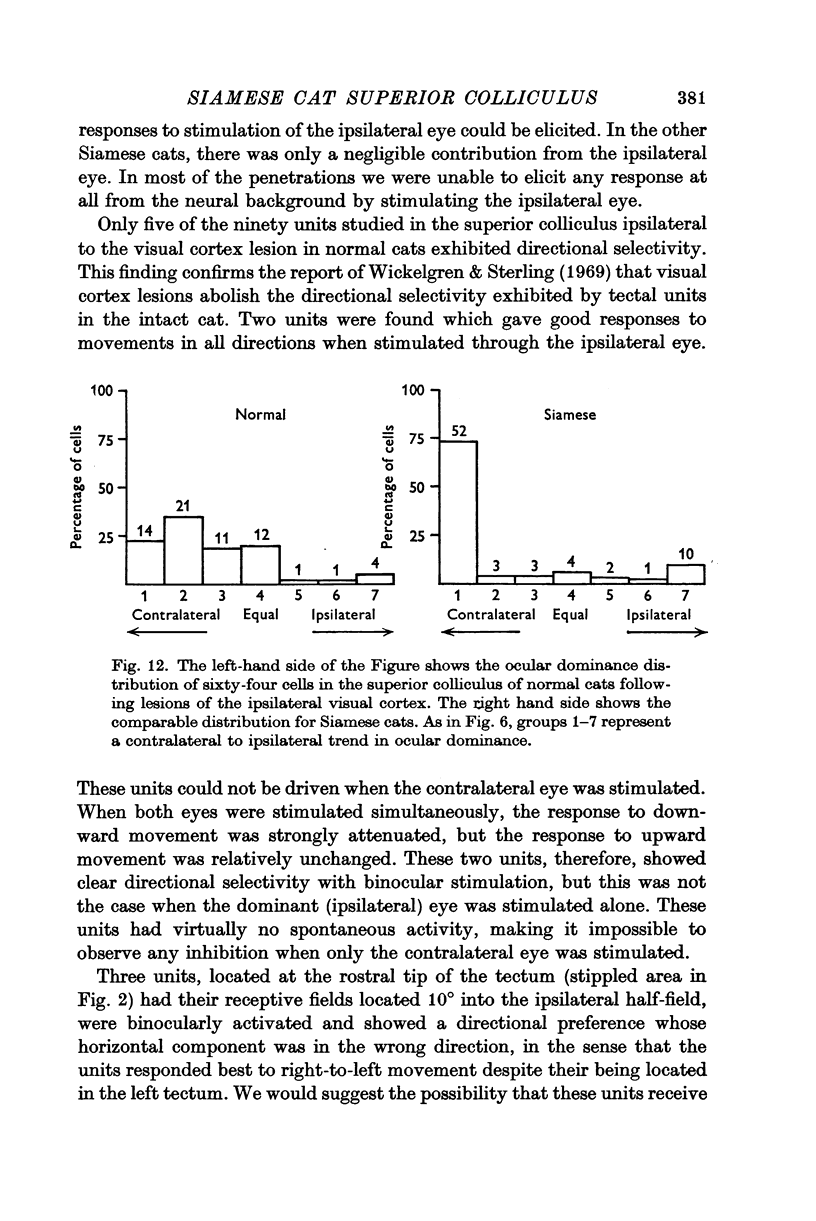
6. Visual cortex lesions in both breeds increase the responsiveness of tectal units to flashing spots and almost entirely remove the directional selectivity exhibited by tectal units, although units with asymmetric surrounds are still found. In normal cats, the lesions change the ocular dominance distribution, skewing it more strongly toward the contralateral eye. In Siamese cats, the ocular dominance distribution remains unchanged after a visual cortex lesion.
7. The squint commonly exhibited by Siamese cats is regarded as a compensation for the anomalous retinotectal topography. It is suggested that, in the absence of an adaptive modification, the anomalous retinotectal projection would lead to mislocalization in Siamese cats just as it does in frogs and hamsters whose retinotectal projection has been experimentally altered. The convergent strabismus which Siamese cats commonly exhibit may be a cure for the abnormal retinal projections rather than a disease.
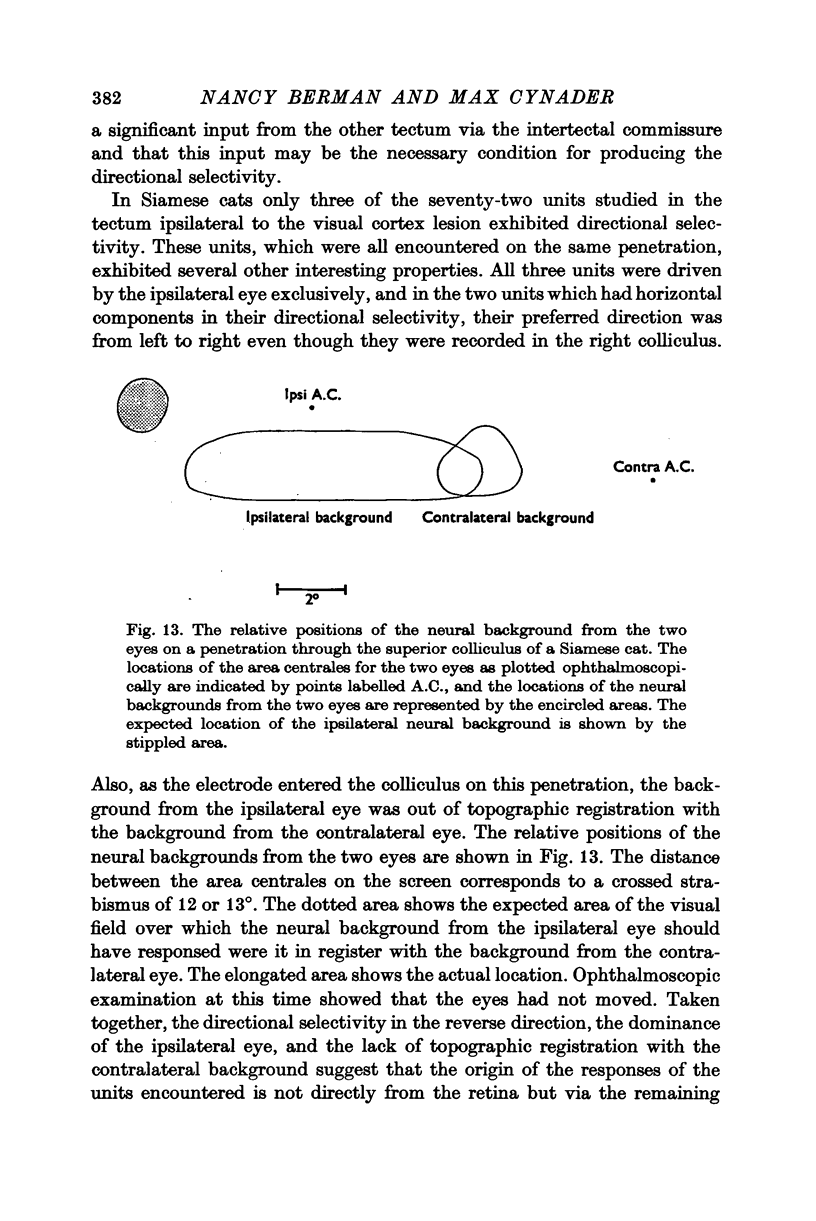
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARE M. H., BISHOP G. H. Responses from an association area secondarily activated from optic cortex. J Neurophysiol. 1954 May;17(3):271–277. doi: 10.1152/jn.1954.17.3.271. [DOI] [PubMed] [Google Scholar]
- Cynader M., Berman N. Receptive-field organization of monkey superior colliculus. J Neurophysiol. 1972 Mar;35(2):187–201. doi: 10.1152/jn.1972.35.2.187. [DOI] [PubMed] [Google Scholar]
- DENNY-BROWN D. The midbrain and motor integration. Proc R Soc Med. 1962 Jul;55:527–538. [PMC free article] [PubMed] [Google Scholar]
- Feldon P., Kruger L. Topography of the retinal projection upon the superior colliculus of the cat. Vision Res. 1970 Feb;10(2):135–143. doi: 10.1016/0042-6989(70)90111-2. [DOI] [PubMed] [Google Scholar]
- Fink R. P., Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967 Apr;4(4):369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Jones E. G., Powell T. P. Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway. J Neurol Neurosurg Psychiatry. 1968 Apr;31(2):135–157. doi: 10.1136/jnnp.31.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. The projection of the retina in the cat. J Anat. 1968 Jan;102(Pt 2):189–222. [PMC free article] [PubMed] [Google Scholar]
- Gaze R. M., Keating M. J., Straznicky K. The re-establishment of retinotectal projections after uncrossing the optic chiasma in Xenopus laevis with one compound eye. J Physiol. 1970 Apr;207(2):51P–52P. [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Aberrant visual projections in the Siamese cat. J Physiol. 1971 Oct;218(1):33–62. doi: 10.1113/jphysiol.1971.sp009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Development of neuronal specificity in retinal ganglion cells of Xenopus. Dev Biol. 1968 Feb;17(2):202–218. doi: 10.1016/0012-1606(68)90061-4. [DOI] [PubMed] [Google Scholar]
- Kalil R. E., Jhaveri S. R., Richards W. Anomalous retinal pathways in the Siamese cat: an inadequate substrate for normal bioncular vision. Science. 1971 Oct 15;174(4006):302–305. doi: 10.1126/science.174.4006.302. [DOI] [PubMed] [Google Scholar]
- Laties A. M., Sprague J. M. The projection of optic fibers to the visual centers in the cat. J Comp Neurol. 1966 May;127(1):35–70. doi: 10.1002/cne.901270104. [DOI] [PubMed] [Google Scholar]
- Leicester J. Projection of the visual vertical meridian to cerebral cortex of the cat. J Neurophysiol. 1968 May;31(3):371–382. doi: 10.1152/jn.1968.31.3.371. [DOI] [PubMed] [Google Scholar]
- Marchiafava P. L., Pepeu G. The responses of units in the superior colliculus of the cat to a moving visual stimulus. Experientia. 1966 Jan 15;22(1):51–53. doi: 10.1007/BF01897768. [DOI] [PubMed] [Google Scholar]
- McIlwain J. T., Buser P. Receptive fields of single cells in the cat's superior colliculus. Exp Brain Res. 1968;5(4):314–325. doi: 10.1007/BF00235906. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Tradardi V., Camarda R. Unit responses to visual stimuli in the cat's superior colliculus after removal of the visual cortex. Brain Res. 1970 Dec 1;24(2):336–339. doi: 10.1016/0006-8993(70)90114-9. [DOI] [PubMed] [Google Scholar]
- Rosenquist A. C., Palmer L. A. Visual receptive field properties of cells of the superior colliculus after cortical lesions in the cat. Exp Neurol. 1971 Dec;33(3):629–652. doi: 10.1016/0014-4886(71)90133-6. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRAGUE J. M., MEIKLE T. H., Jr THE ROLE OF THE SUPERIOR COLLICULUS IN VISUALLY GUIDED BEHAVIOR. Exp Neurol. 1965 Jan;11:115–146. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Sherman S. M. Nasotemporal overlap in visual field projected to lateral geniculate nucleus in the cat. J Neurophysiol. 1971 May;34(3):453–466. doi: 10.1152/jn.1971.34.3.453. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Mechanisms of functional recovery following lesions of visual cortex or superior colliculus in neonate and adult hamsters. Brain Behav Evol. 1970;3(1):295–323. doi: 10.1159/000125479. [DOI] [PubMed] [Google Scholar]
- Sterling P., Wickelgren B. G. Visual receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):1–15. doi: 10.1152/jn.1969.32.1.1. [DOI] [PubMed] [Google Scholar]
- Straschill M., Hoffmann K. P. Functional aspects of localization in the cat's tectum opticum. Brain Res. 1969 Apr;13(2):274–283. doi: 10.1016/0006-8993(69)90287-x. [DOI] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]