Abstract
1. A delayed current decrease associated with prolonged depolarization was studied in R15 (the parabolic burster) of Aplysia by using intracellular recording and voltage clamp techniques.
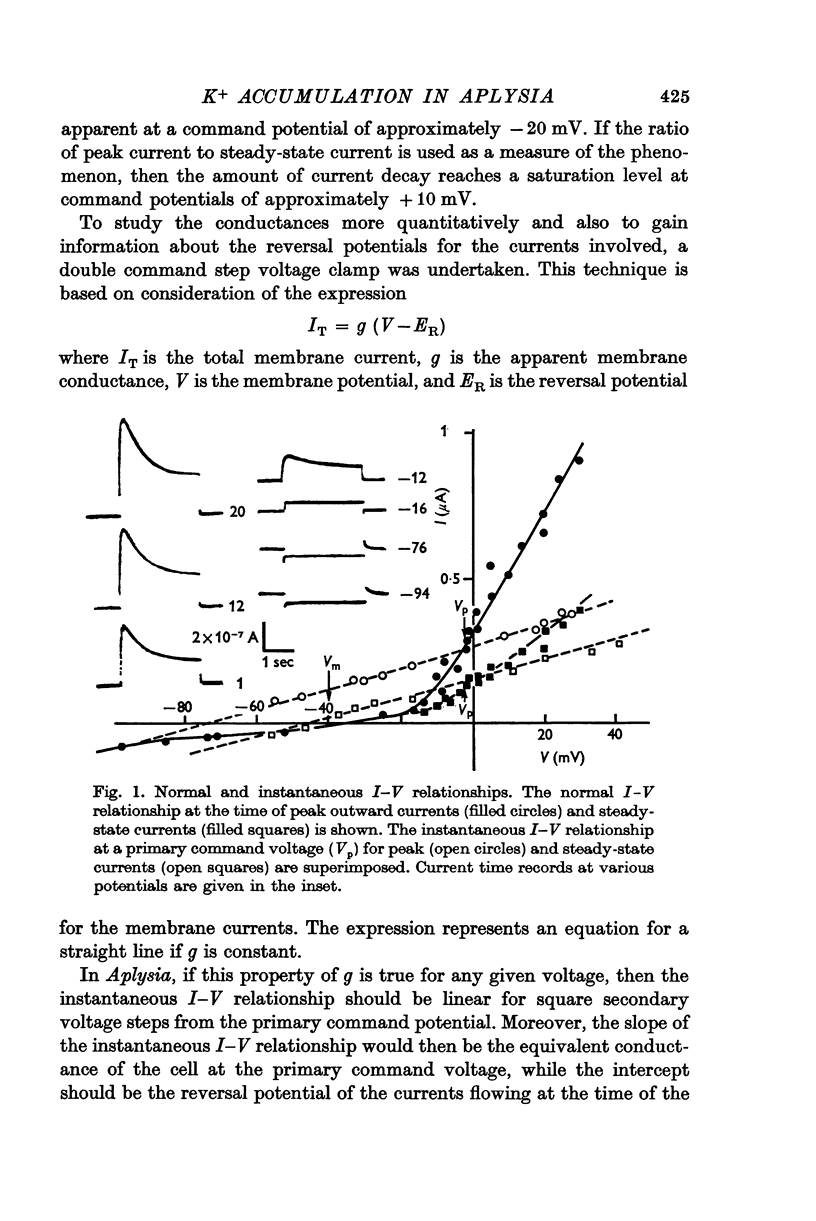
2. For long duration command pulses (3 sec), the outward current shows a delayed decrease. The current goes from a maximum near 100 msec and falls until a steady-state outward current is reached between 1·5 and 2·5 sec after the beginning of the command step. This final steady-state current is usually only about 20-30% of the peak outward current.
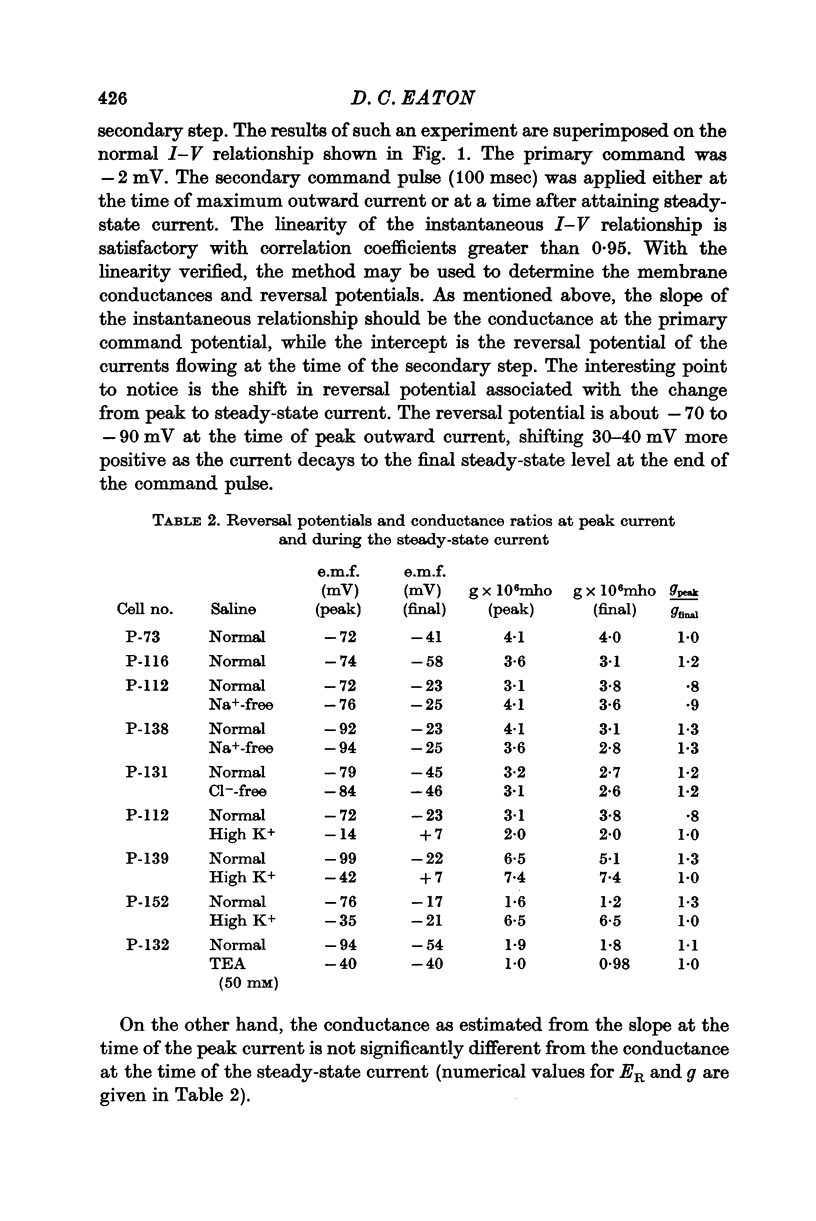
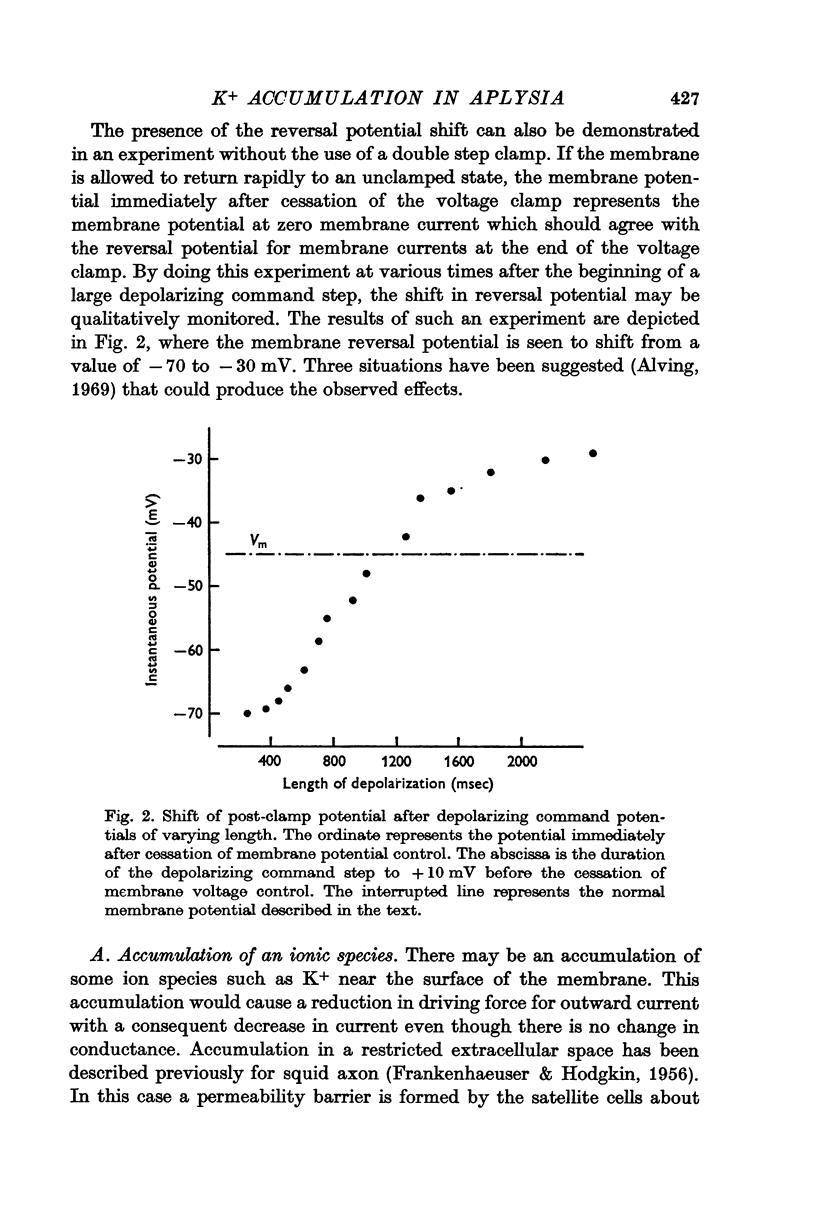
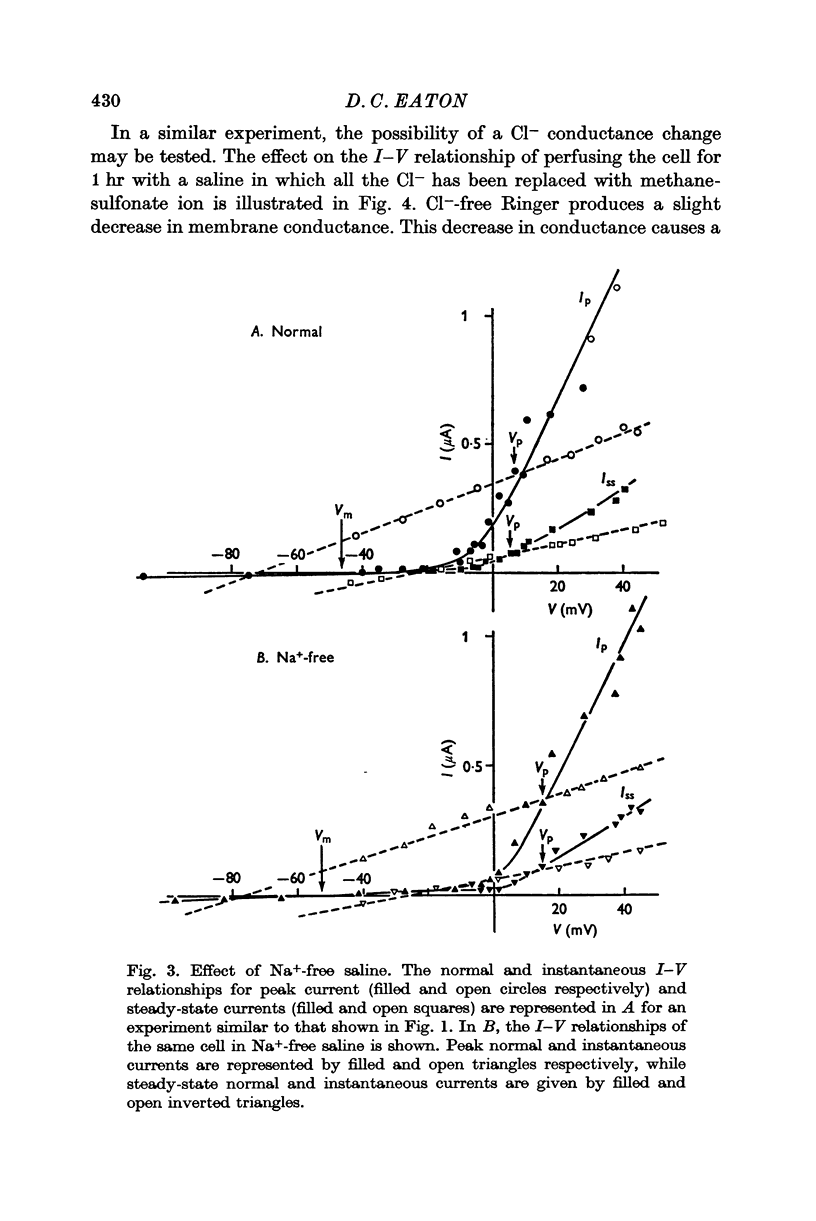
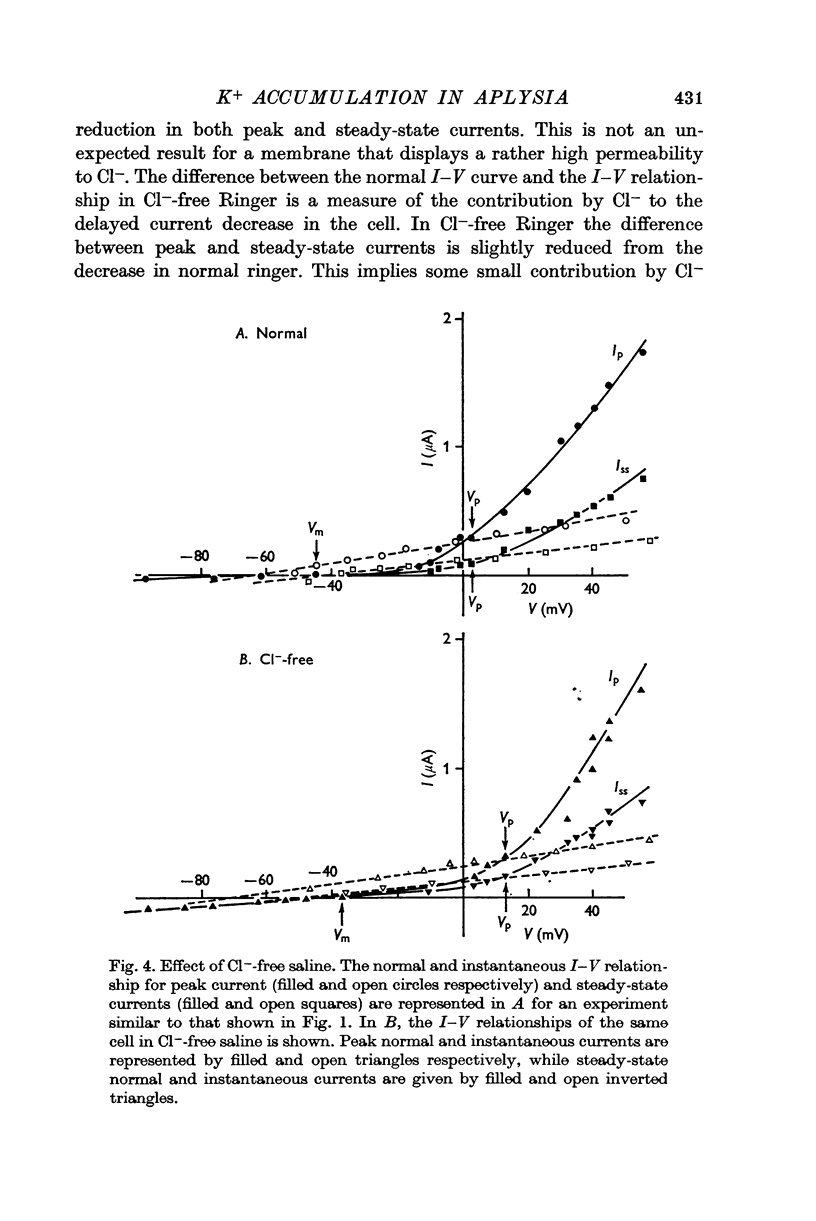
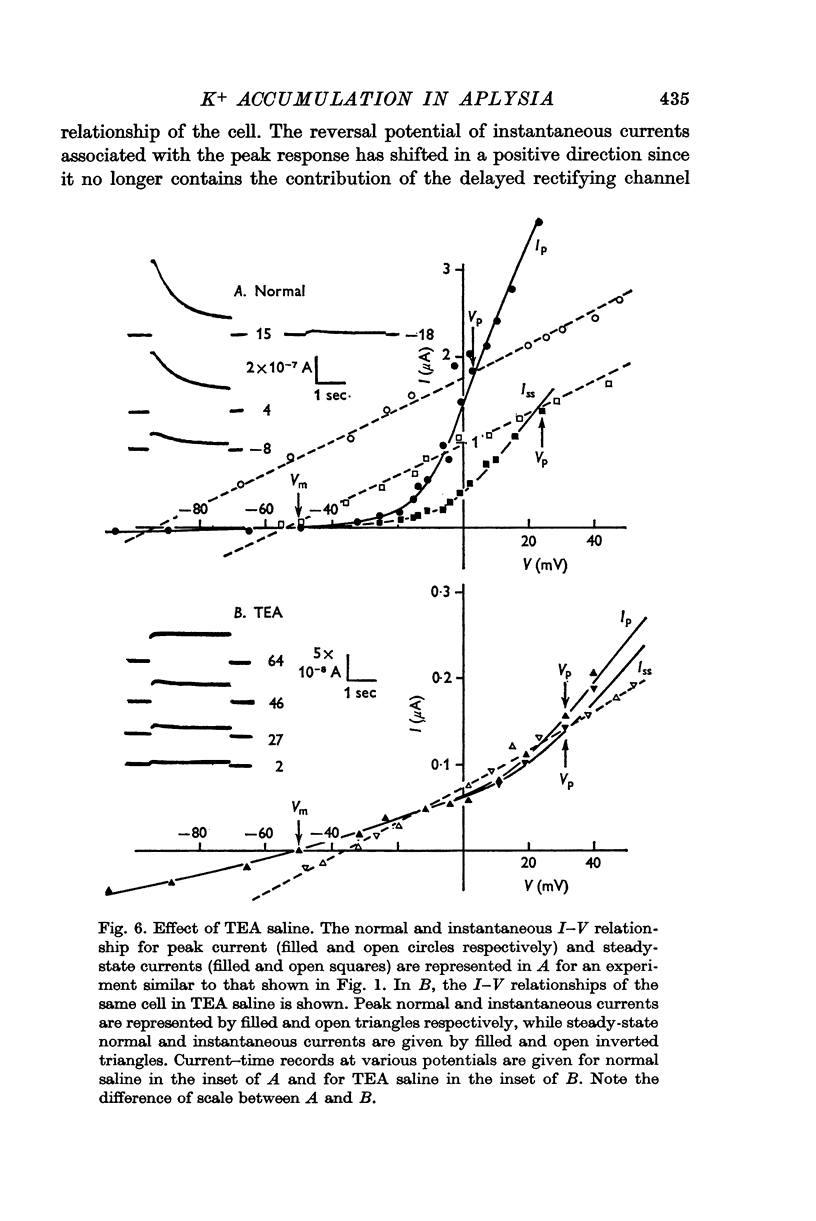
3. Double step voltage clamps show that this current decrease is associated with a large shift of e.m.f. Measurements of conductance, on the other hand, fail to show any significant difference in conductance associated with peak and steady-state currents.
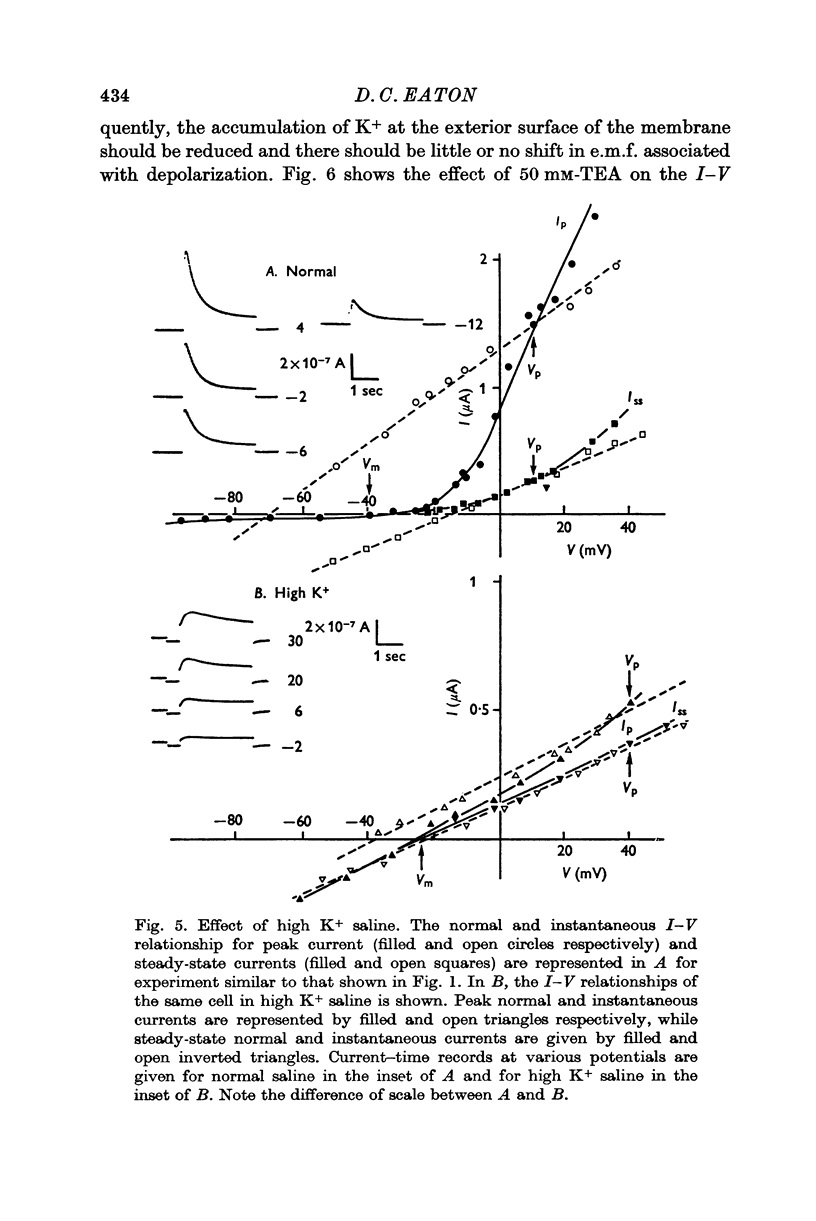
4. From the results of application of high K+ ringer, the conclusion is reached that this shift in e.m.f. is due to an accumulation of K+ near the exterior cell membrane. Several other experiments exclude the possibility of either metabolic events or compensating conductance changes producing the phenomenon.
5. The location of the accumulation is considered on the basis of anatomical studies. It is concluded that the accumulation takes place in the extensive infoldings found in cells like R15. An explanation of the difference in delayed current decrease between pace-makers and non-pace-makers is suggested, since the pace-makers apparently have more extensive invaginations than the non-pace-makers. This suggestion is lent support by measurements of capitance and current density.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving B. O. Differences between pacemaker and nonpacemaker neurons of Aplysia on voltage clamping. J Gen Physiol. 1969 Oct;54(4):512–531. doi: 10.1085/jgp.54.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E. A light and electron microscope study of the abdominal ganglion of Aplysia californica. J Neurophysiol. 1967 Nov;30(6):1263–1287. doi: 10.1152/jn.1967.30.6.1263. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geren B. B., Schmitt F. O. THE STRUCTURE OF THE SCHWANN CELL AND ITS RELATION TO THE AXON IN CERTAIN INVERTEBRATE NERVE FIBERS. Proc Natl Acad Sci U S A. 1954 Sep;40(9):863–870. doi: 10.1073/pnas.40.9.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., SAITO N. Voltage-current relations in nerve cell membrane of Onchidium verruculatum. J Physiol. 1959 Oct;148:161–179. doi: 10.1113/jphysiol.1959.sp006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K., Junge D. Excitation-contraction coupling in a barnacle muscle fiber as examined with voltage clamp technique. J Gen Physiol. 1968 Feb;51(2):157–175. doi: 10.1085/jgp.51.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIKUCHI R., NAITO K., TANAKA I. Effect of sodium and potassium ions on the electrical activity of single cells in the lateral eye of the horseshoe crab. J Physiol. 1962 May;161:319–343. doi: 10.1113/jphysiol.1962.sp006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBLUTH J. The visceral ganglion of Aplysia californica. Z Zellforsch Mikrosk Anat. 1963;60:213–236. doi: 10.1007/BF00350477. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I. The sodium-potassium exchange pump: relation of metabolism to electrical properties of the cell. I. Theory. Biophys J. 1970 Mar;10(3):246–259. doi: 10.1016/S0006-3495(70)86297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G. On the mechanism of spontaneous impulse generation in the pacemaker of the heart. J Gen Physiol. 1961 Nov;45:317–330. doi: 10.1085/jgp.45.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]


