Abstract
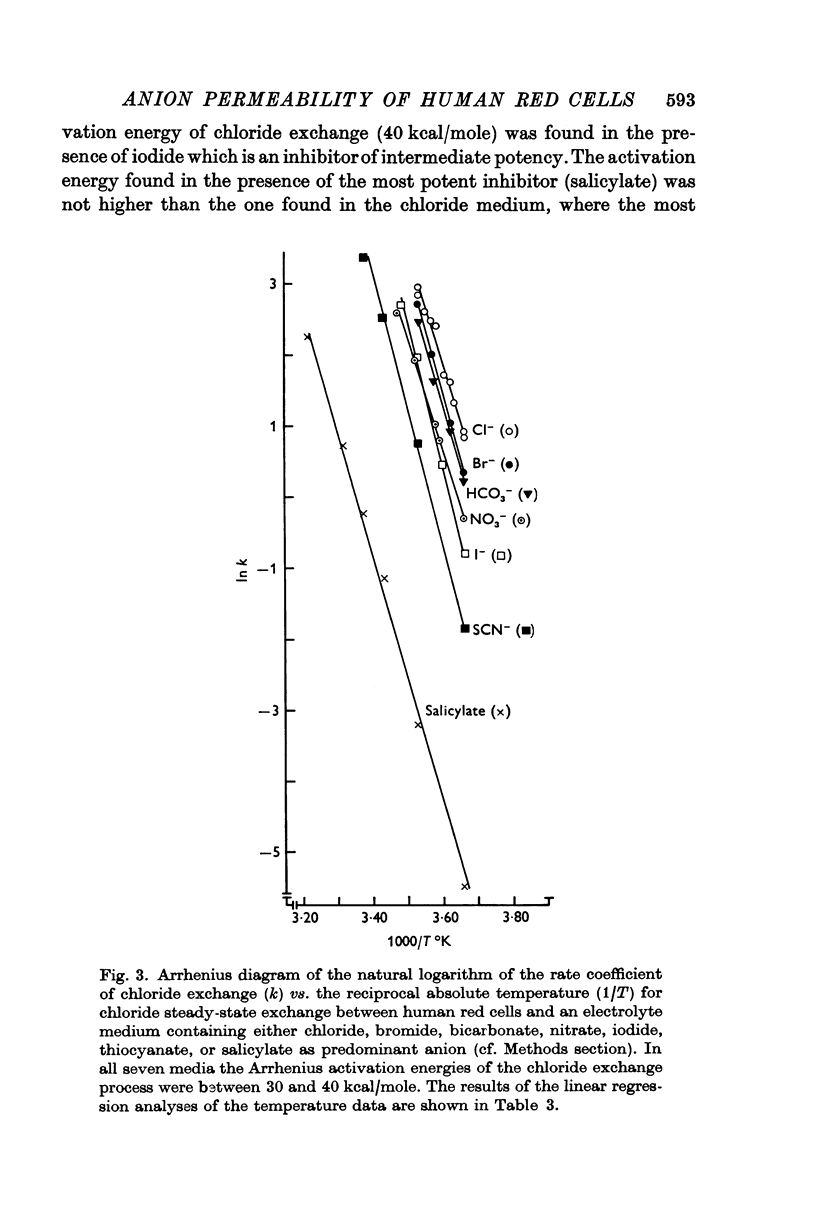
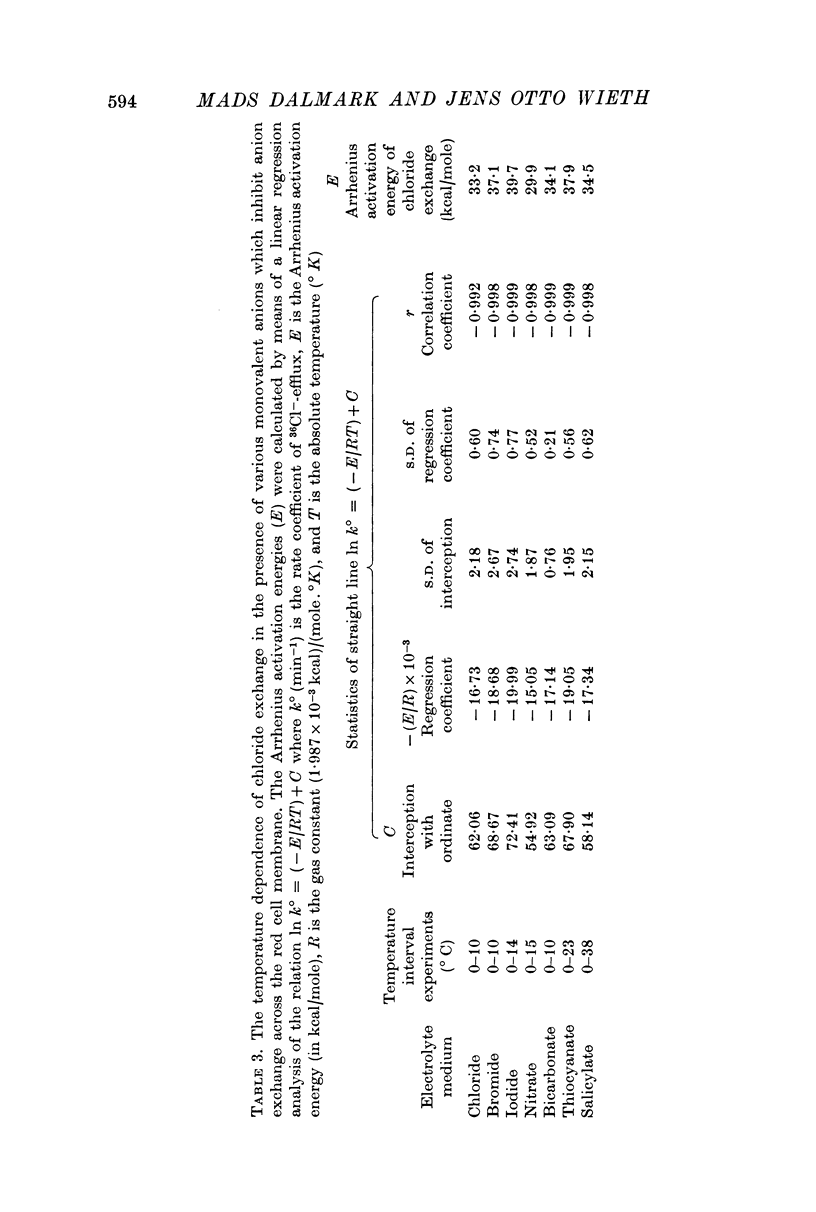
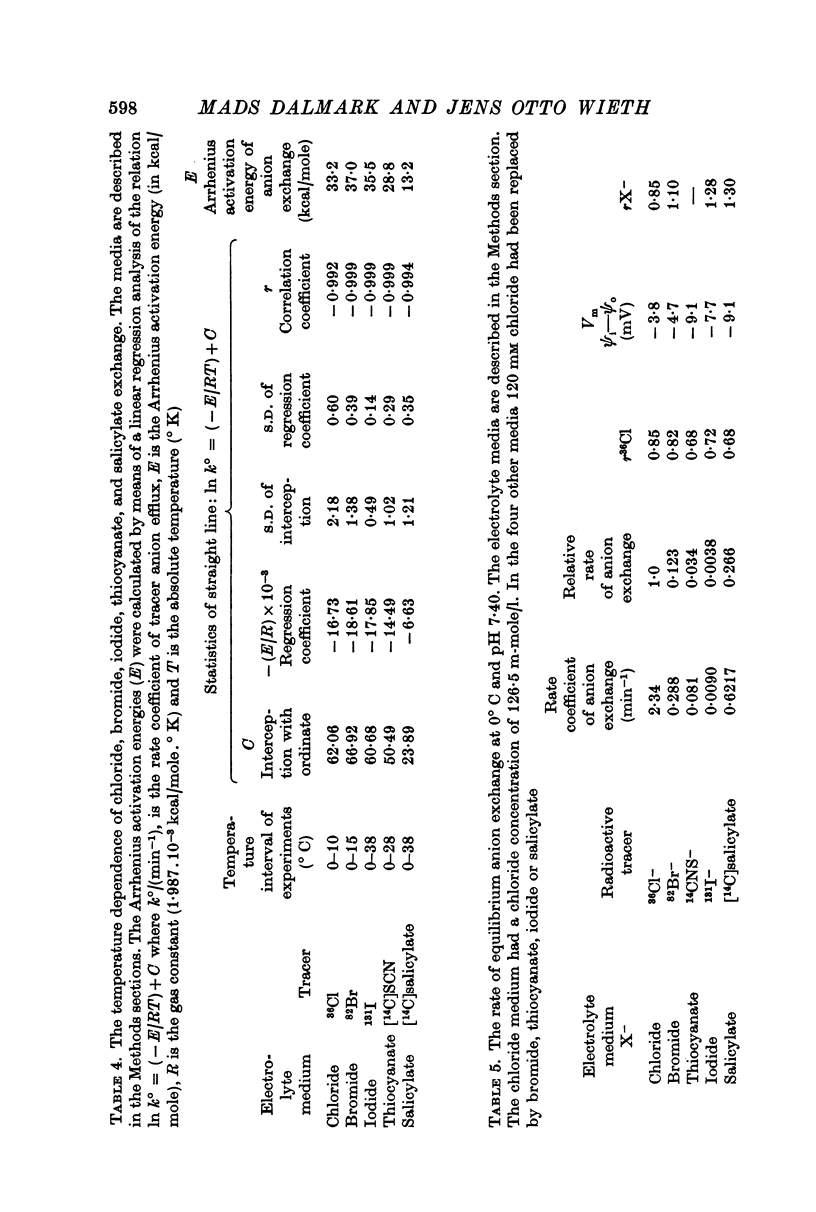
1. The temperature dependence of the steady-state self-exchange of chloride between human red cells and a plasma-like electrolyte medium has been studied by measuring the rate of 36Cl- efflux from radioactively labelled cells. Between 0 and 10° C the rate increased by a factor of eight corresponding to an Arrhenius activation energy of 33 kcal/mole.
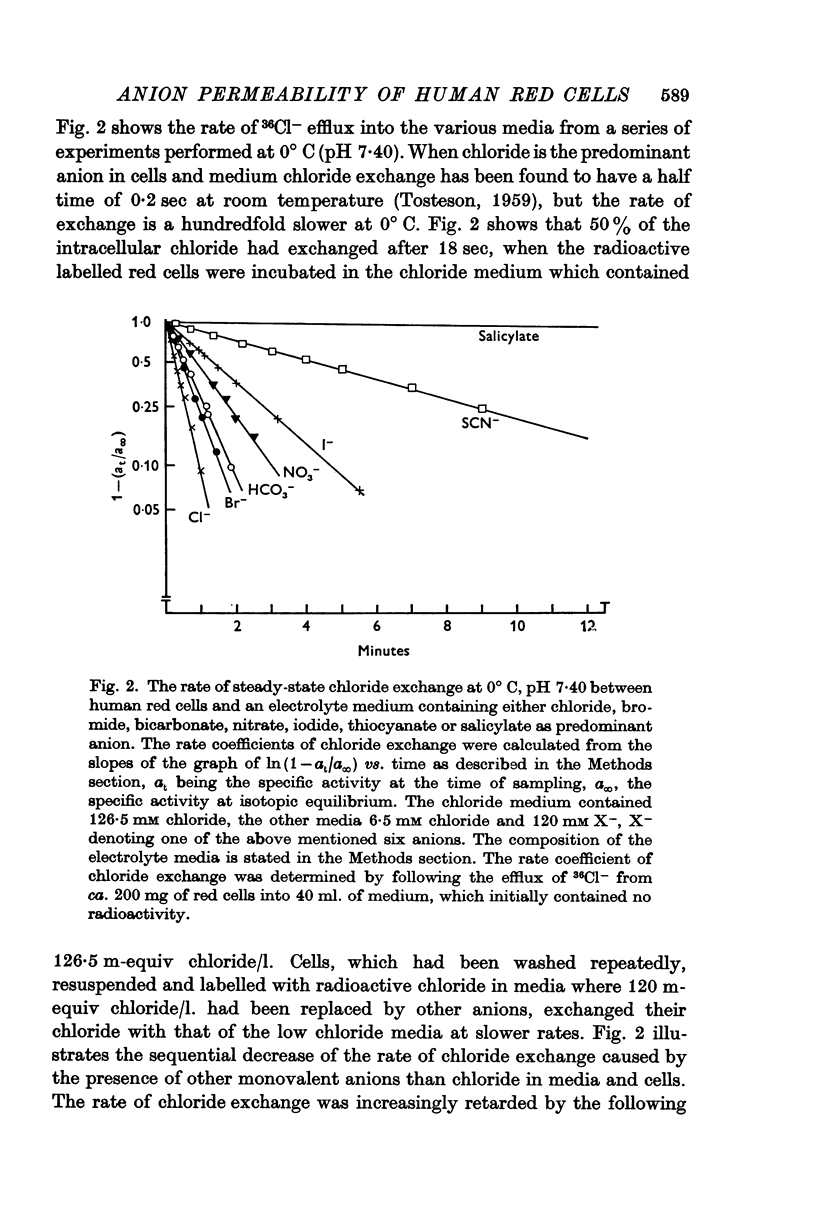
2. The rate of chloride exchange decreased significantly in experiments where 95% of the chloride ions in cells and medium were replaced by other monovalent anions of a lyotropic series. The rate of chloride self-exchange was increasingly reduced by bromide, bicarbonate, nitrate, iodide, thiocyanate, and salicylate. The latter aromatic anion was by far the most potent inhibitor, reducing the rate of chloride self-exchange to 0·2% of the value found in a chloride medium.
3. The temperature sensitivity of the chloride self-exchange was not affected significantly by the anionic inhibitors. The Arrhenius activation energies of chloride exchange were between 30 and 40 kcal/mole in the presence of the six inhibitory anions mentioned above.
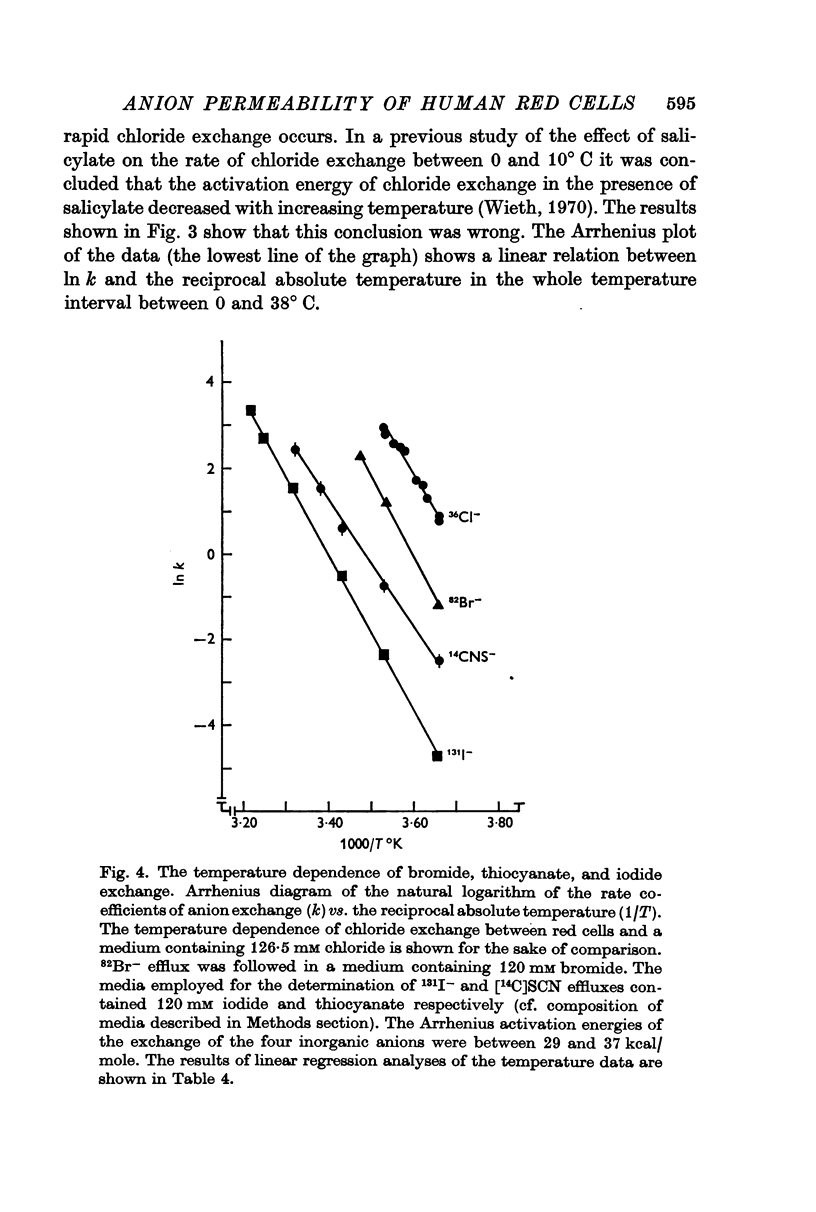
4. The rate of self-exchange of bromide, thiocyanate, and iodide between human red cells and media was determined after washing and labelling cells in media containing 120 mM bromide, thiocyanate, or iodide respectively. The rate of self-exchange of the three anions were 12, 3, and 0·4% of the rate of chloride self-exchange found in the chloride medium.
5. The Arrhenius activation energies of the self-exchange of bromide, iodide, and thiocyanate were all between 29 and 37 kcal/mole, the same magnitude as found for the self-exchange of chloride.
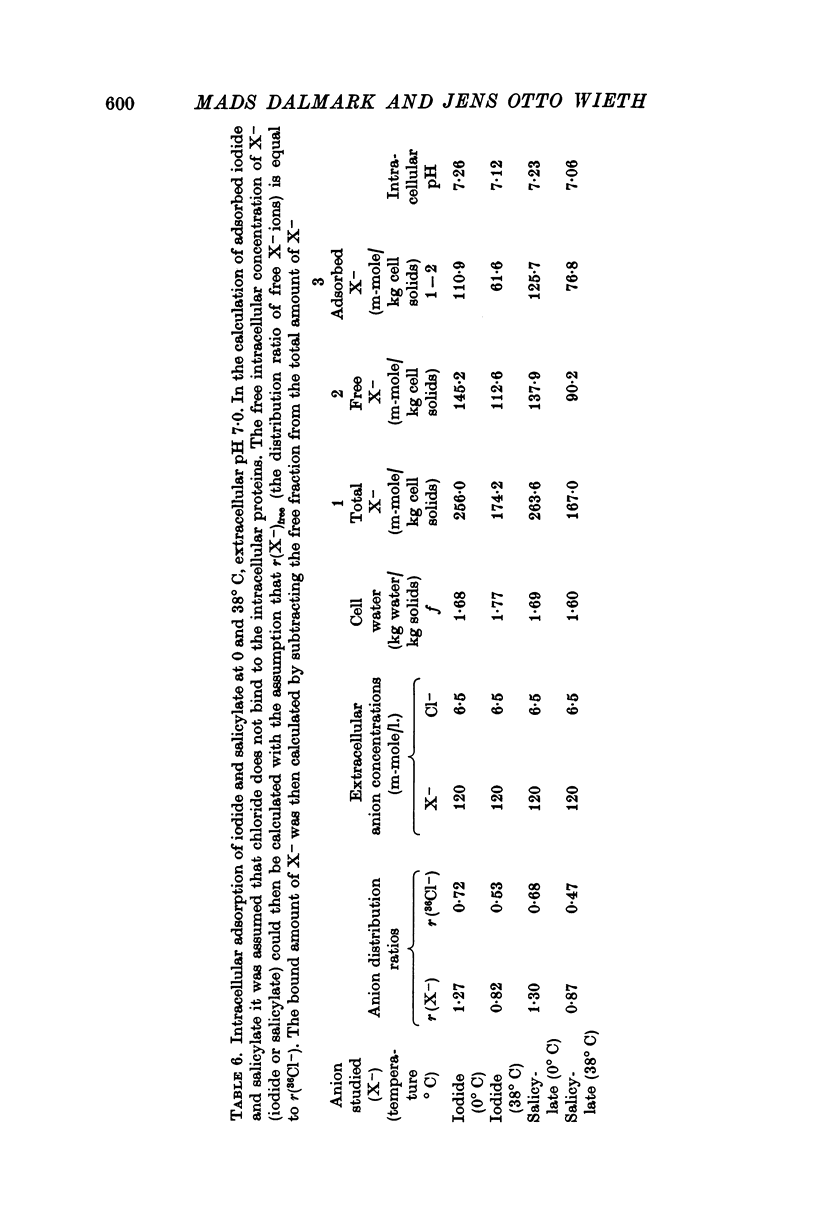
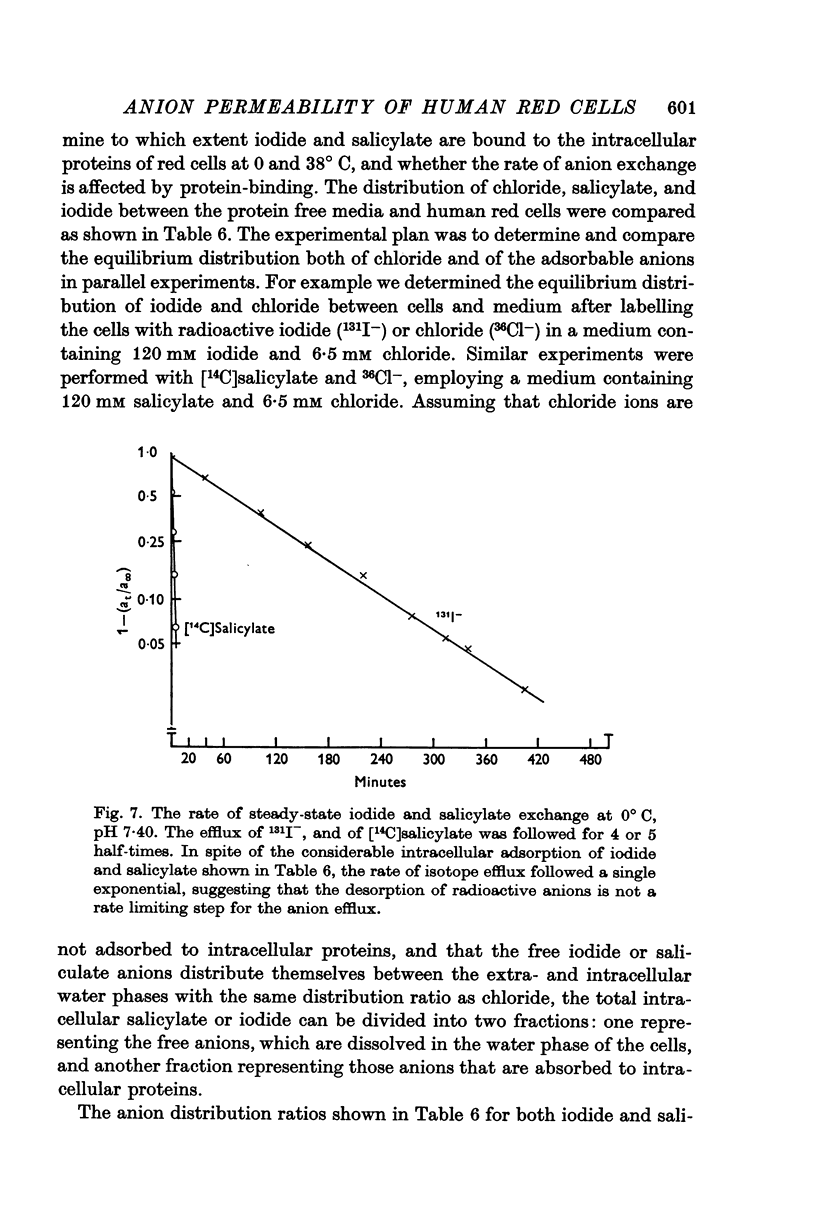
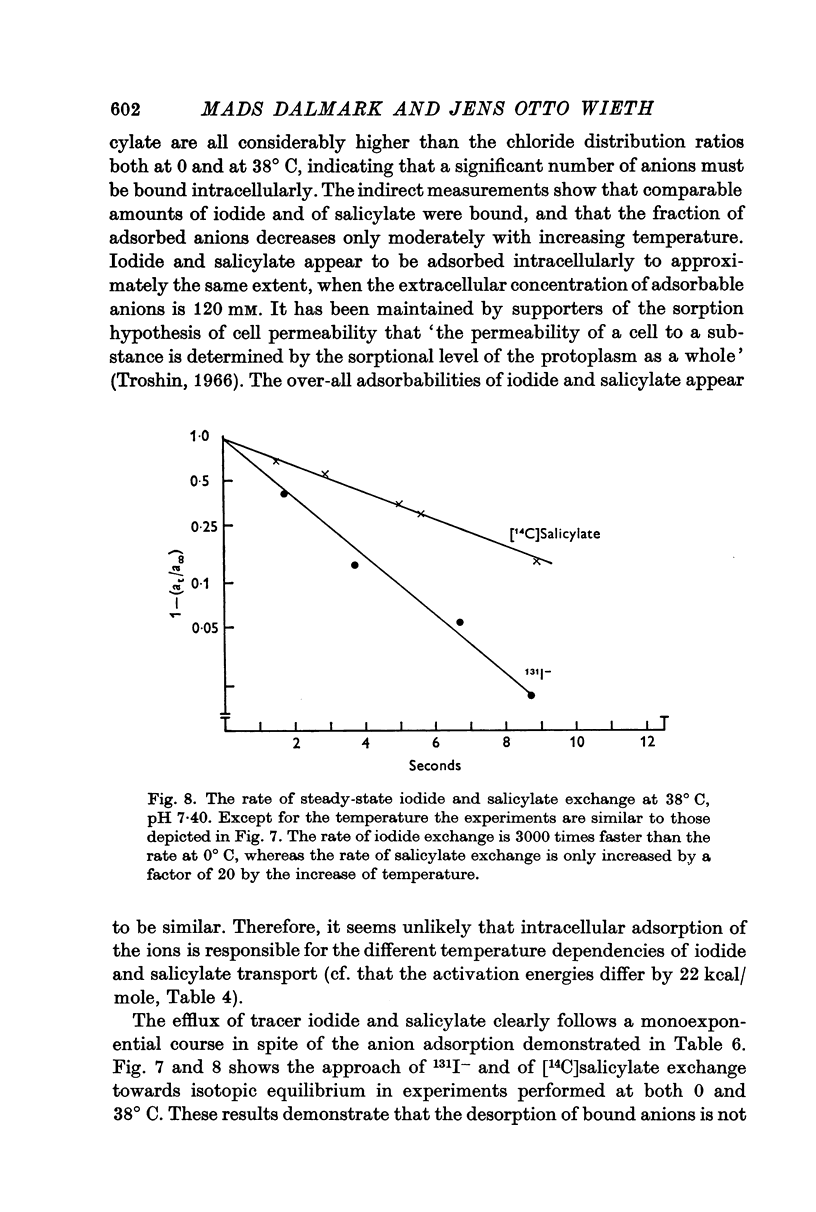
6. Although approximately 40% of the intracellular iodide and salicylate ions appeared to be adsorbed to intracellular proteins, the rate of tracer anion efflux followed first order kinetics until at least 98% of the intracellular anions had been exchanged.
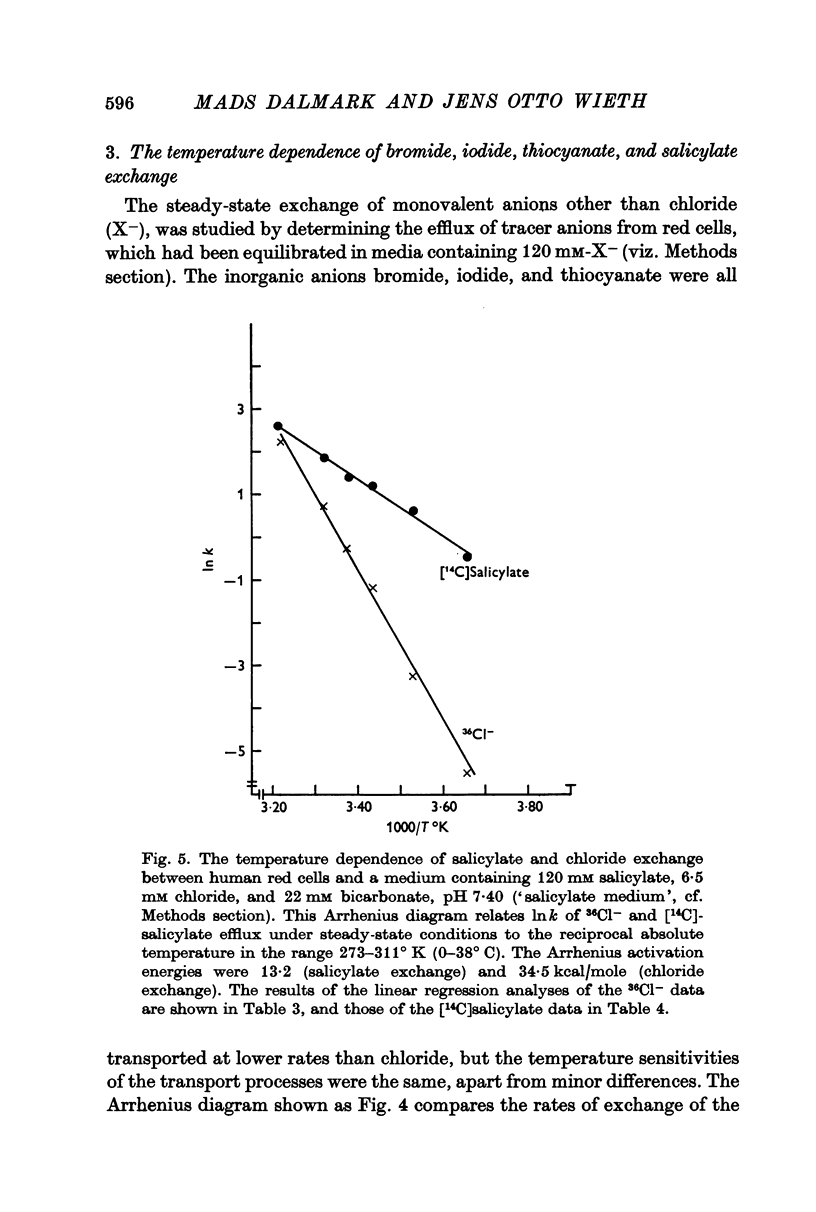
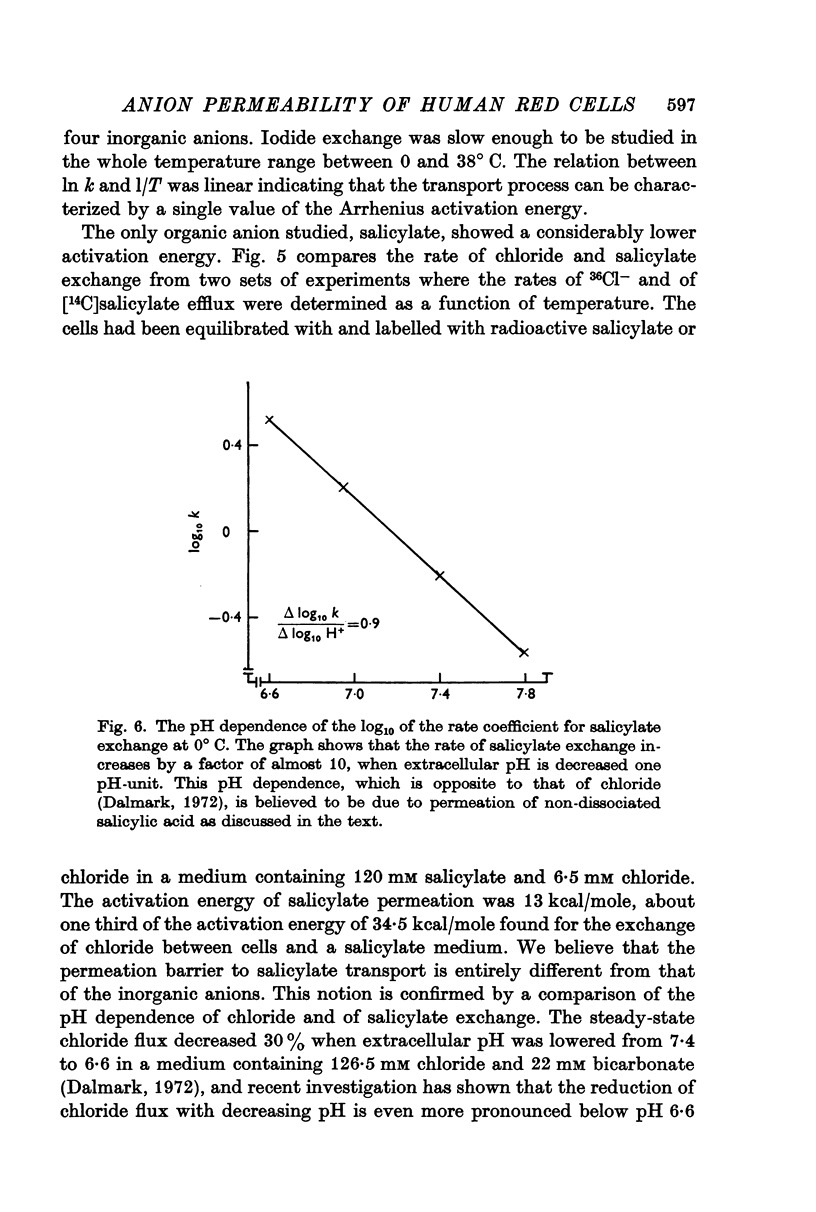
7. The self-exchange of salicylate across the human red cell membrane occurred by a different mechanism than the one utilized by the inorganic monovalent anions. The activation energy of salicylate exchange (13·2 kcal/mole) was significantly lower than that of inorganic anion exchange. Salicylate exchange increased with decreasing pH in contrast to the exchange of chloride, which decreases when pH is lowered.
Full text
PDF


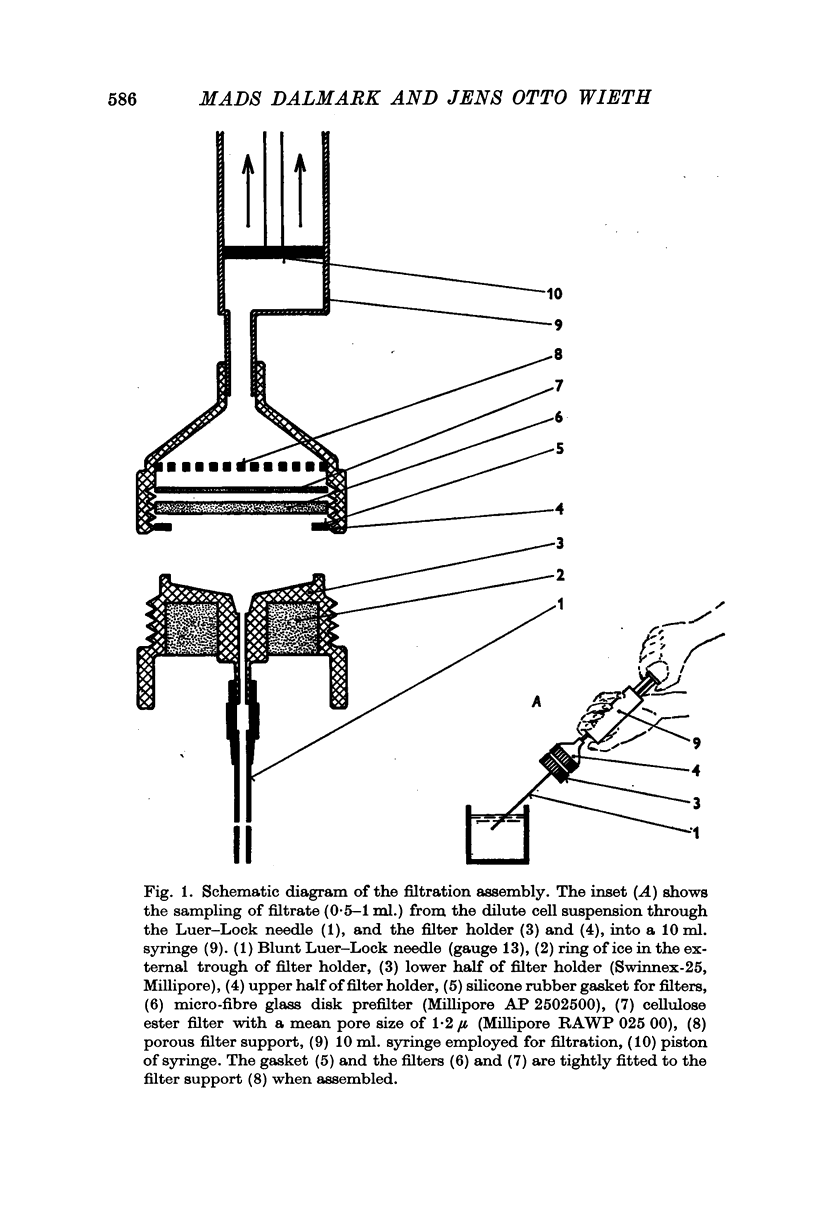














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalmark M., Wieth J. O. Chloride and sodium permeabilities of human red cells. Biochim Biophys Acta. 1970 Dec 1;219(2):525–527. doi: 10.1016/0005-2736(70)90239-7. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Effect of ouabain on gluclose metabolism and on fluxes of sodium and potassium of human blood cells. Acta Physiol Scand. 1967 Sep;71(1):113–124. doi: 10.1111/j.1748-1716.1967.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Trapping of sodium, potassium, sucrose, and albumin in the packed cell column of the hematocrit. Acta Physiol Scand. 1967 Sep;71(1):105–112. doi: 10.1111/j.1748-1716.1967.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Tosteson D. C. The effect of 2,4,6-trinitro-m-cresol on cation and anion transport in sheep red blood cells. J Gen Physiol. 1971 May;57(5):593–609. doi: 10.1085/jgp.57.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Bangham A. D. The action of anaesthetics on phospholipid membranes. Biochim Biophys Acta. 1969 Oct 14;193(1):92–104. doi: 10.1016/0005-2736(69)90062-5. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCKNER H. Die Temperaturabhängigkeit des Anionenaustausches roter Blutkörperchen. Pflugers Arch Gesamte Physiol Menschen Tiere. 1948;250(3):303–311. [PubMed] [Google Scholar]
- Mawe R. C., Hempling H. G. The exchange of C14 glucose across the membrane of the human erythrocyte. J Cell Physiol. 1965 Aug;66(1):95–103. doi: 10.1002/jcp.1030660110. [DOI] [PubMed] [Google Scholar]
- PIIPER J. GESCHWINDIGKEIT DES CO2-AUSTAUSCHES ZWISCHEN ERYTHROCYTEN UND PLASMA. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964;278:500–512. [PubMed] [Google Scholar]
- Passow H. Passive ion permeability of the erythrocyte membrane. Prog Biophys Mol Biol. 1969;19(2):423–467. [PubMed] [Google Scholar]
- Passow H., Schnell K. F. Chemical modifiers of passive ion permeability of the erythrocyte membrane. Experientia. 1969 May 15;25(5):460–468. doi: 10.1007/BF01900757. [DOI] [PubMed] [Google Scholar]
- Wieth J. O. Effect of some monovalent anions on chloride and sulphate permeability of human red cells. J Physiol. 1970 May;207(3):581–609. doi: 10.1113/jphysiol.1970.sp009082. [DOI] [PMC free article] [PubMed] [Google Scholar]


