Abstract
1. The action of adenosine on neuromuscular transmission has been studied on the rat phrenic nerve—diaphragm preparation.
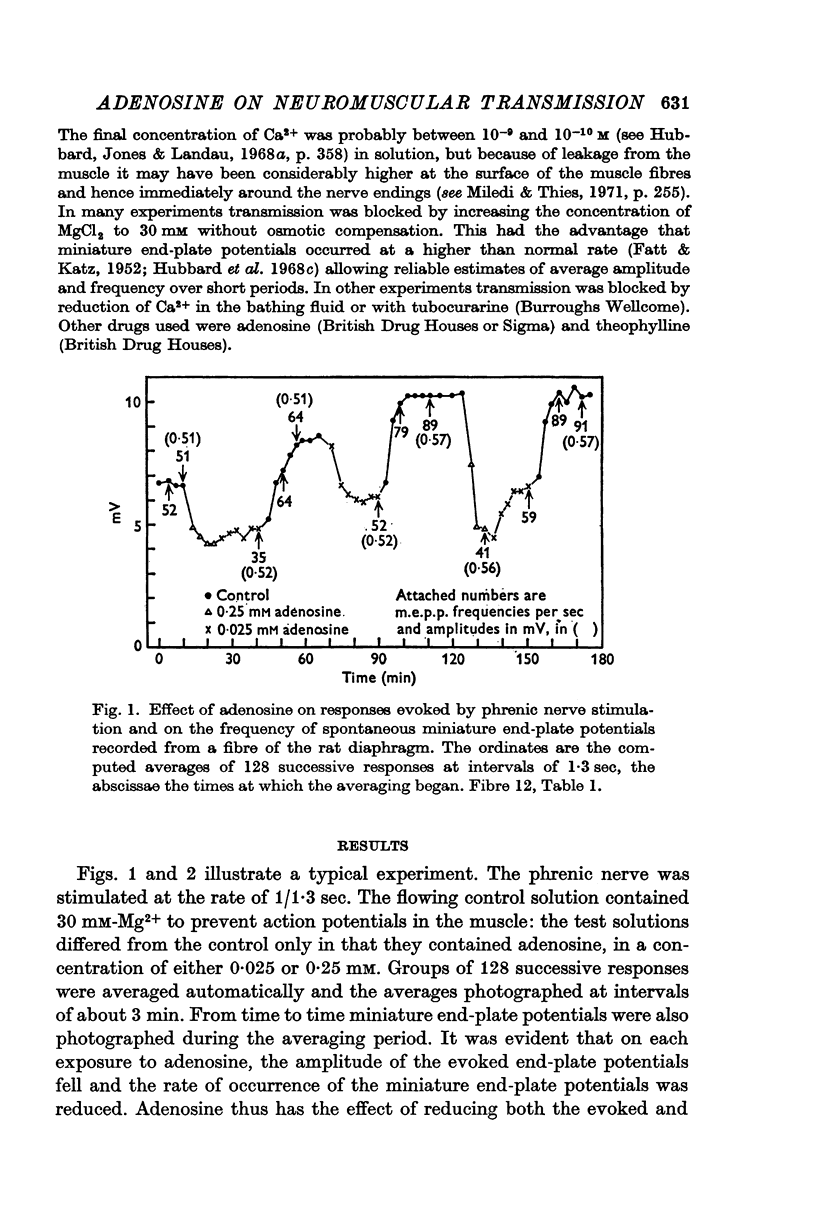
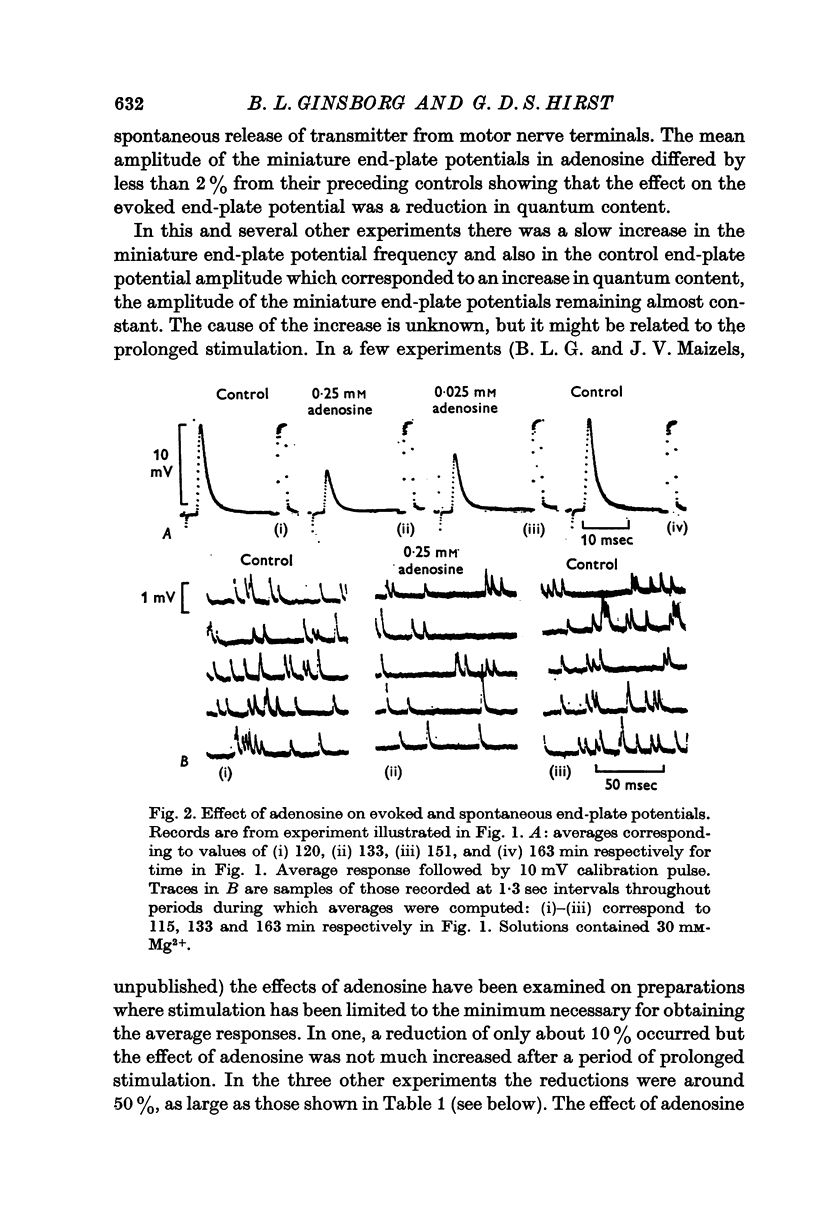
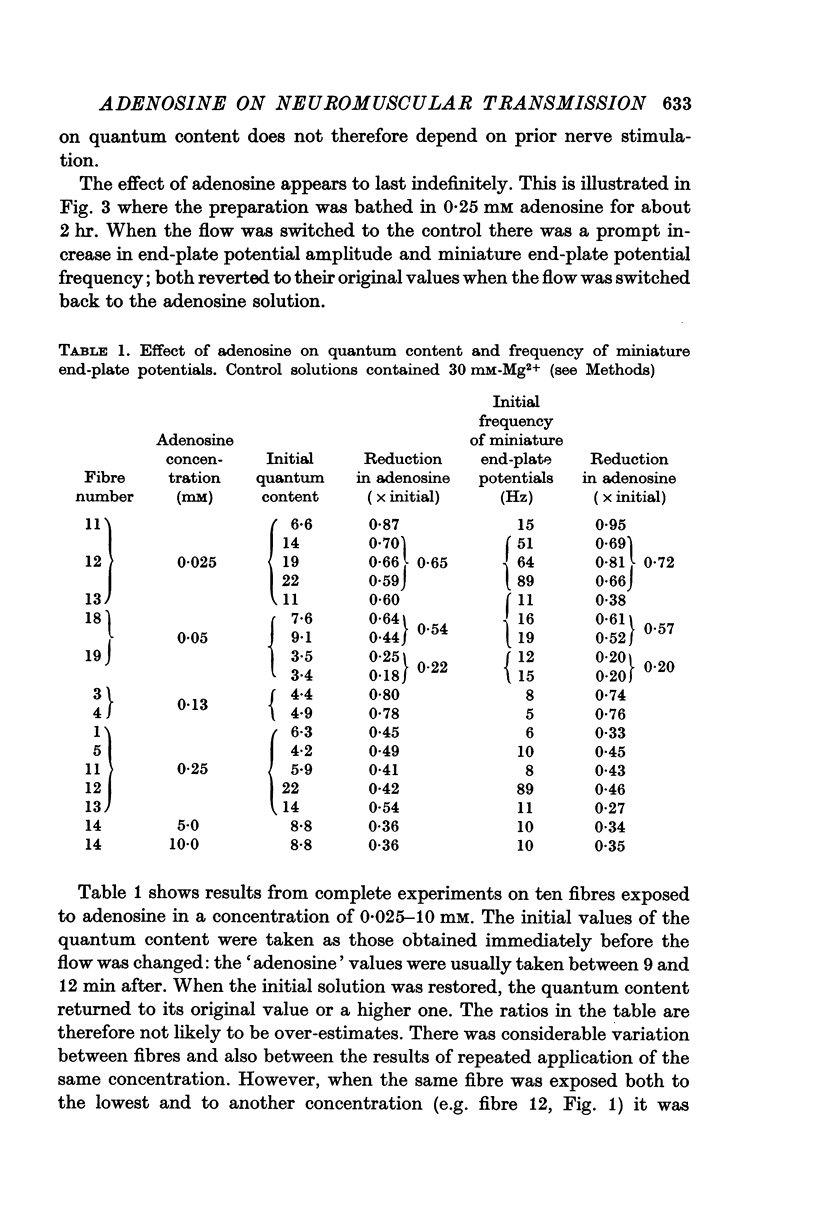
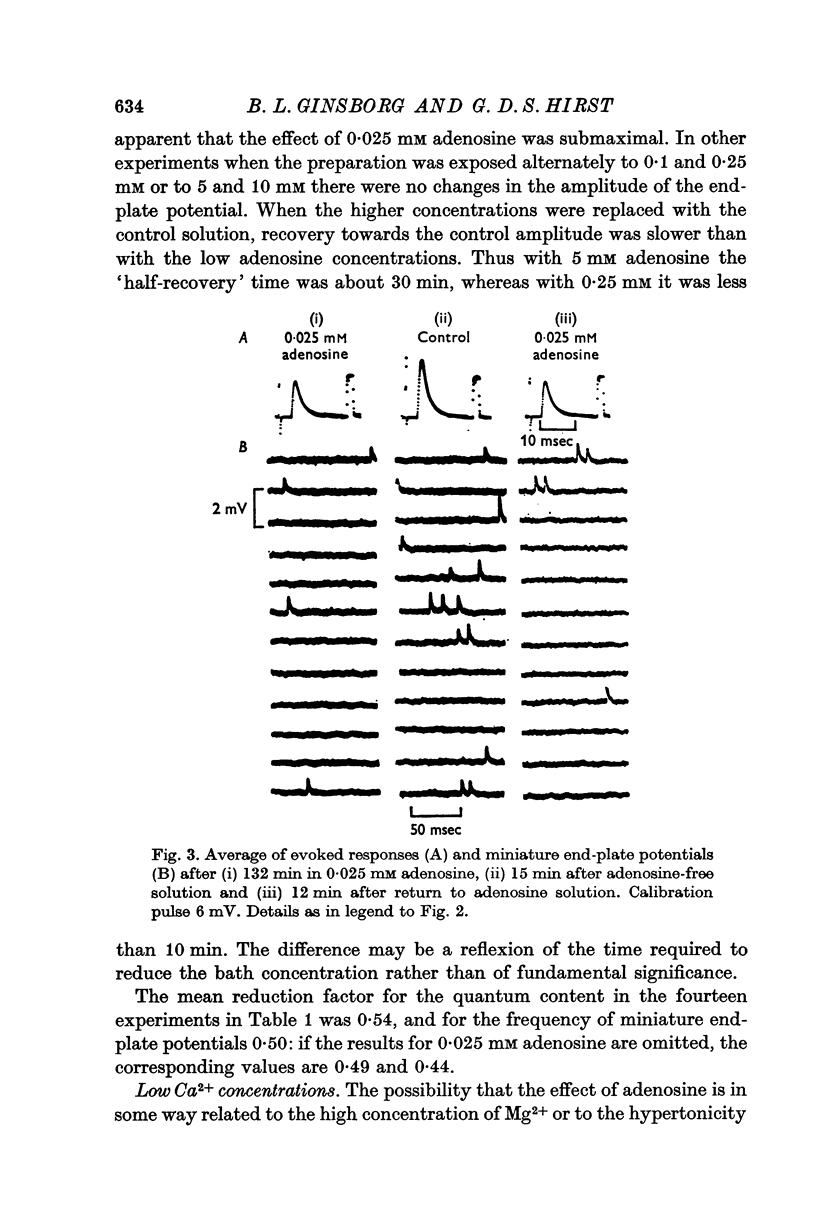
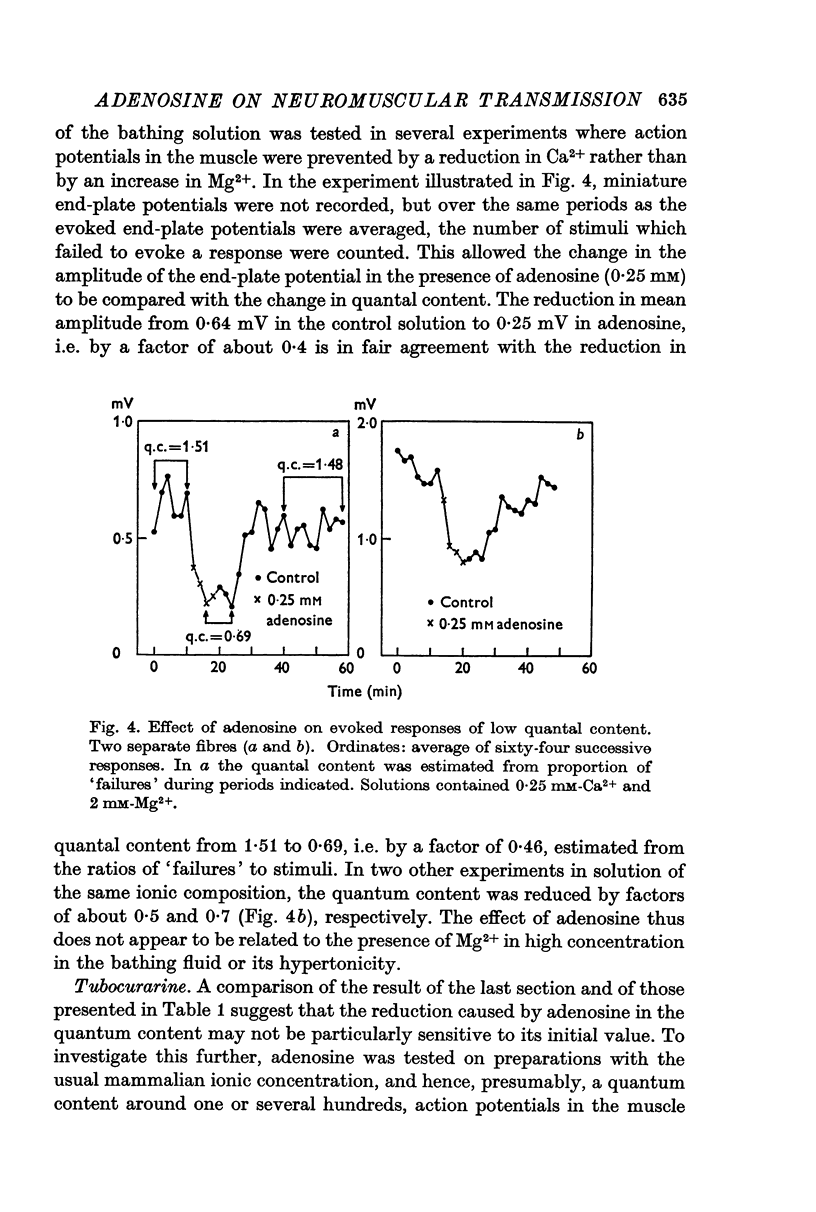
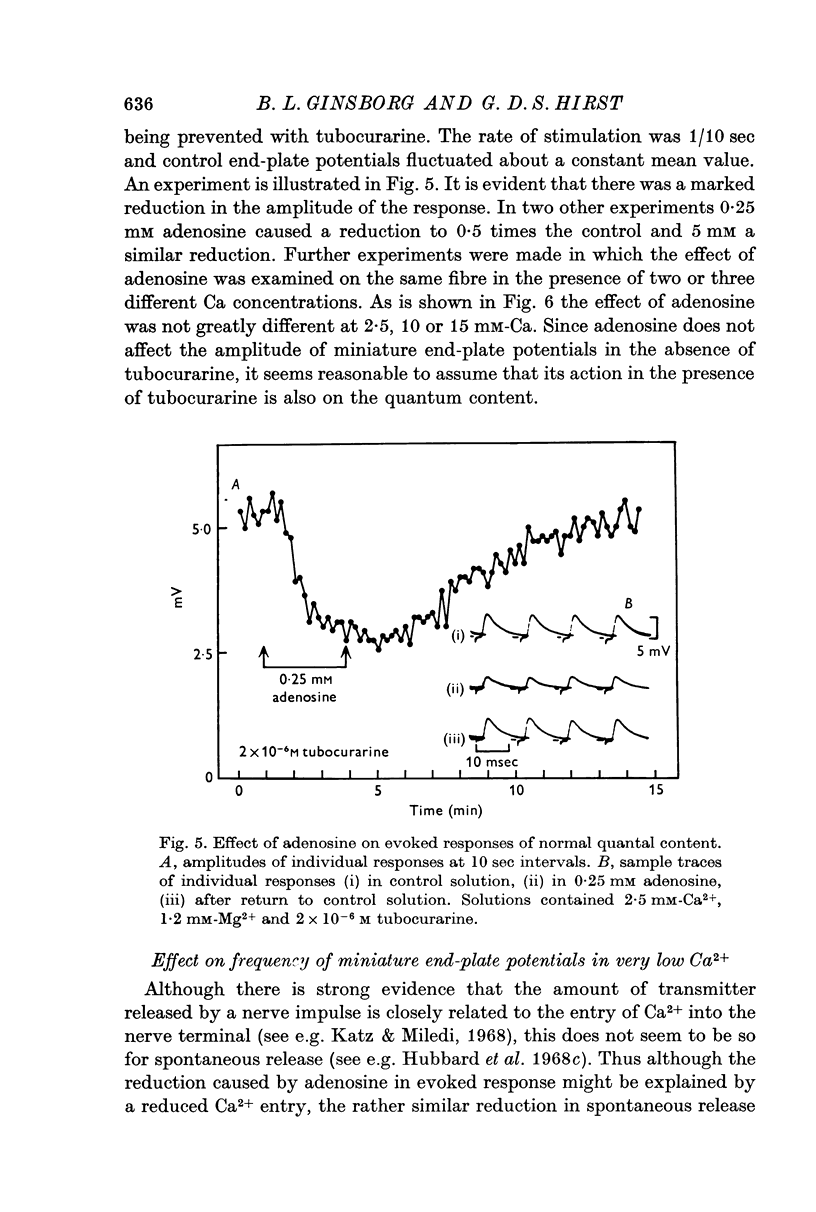
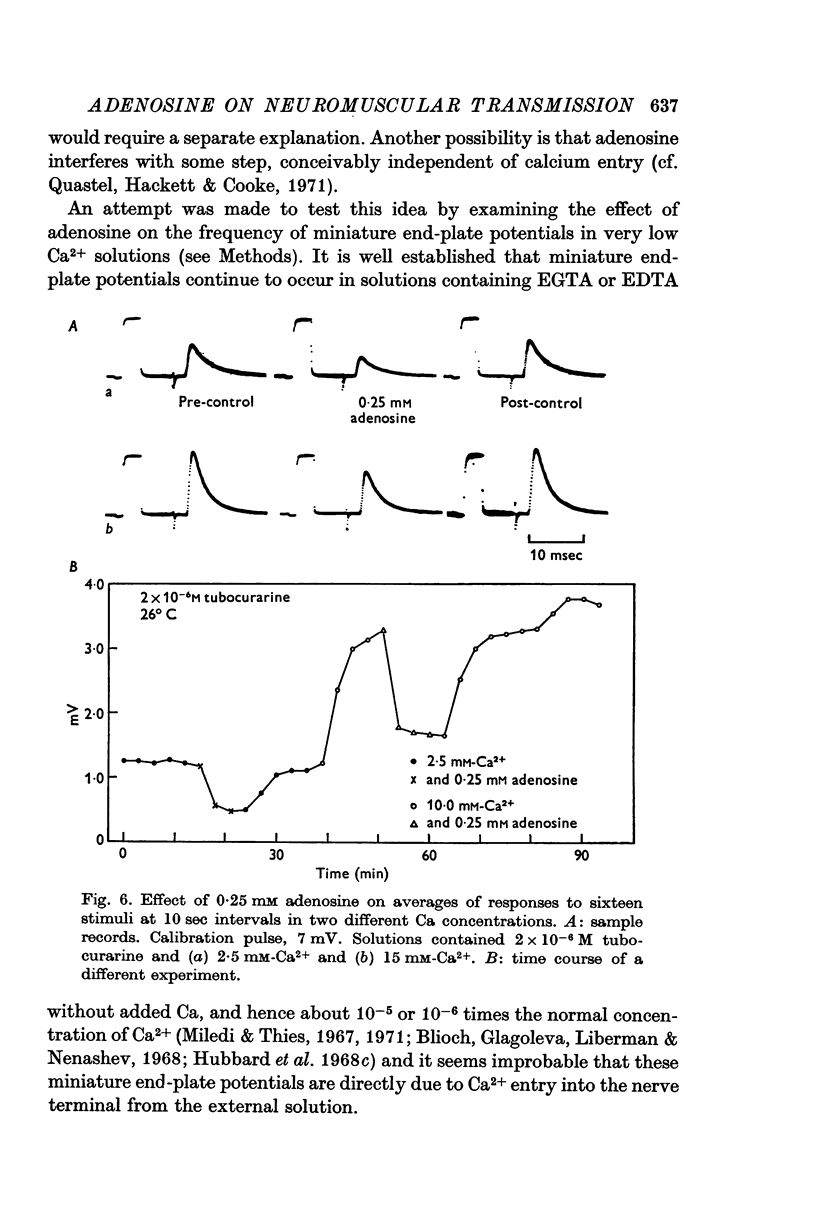
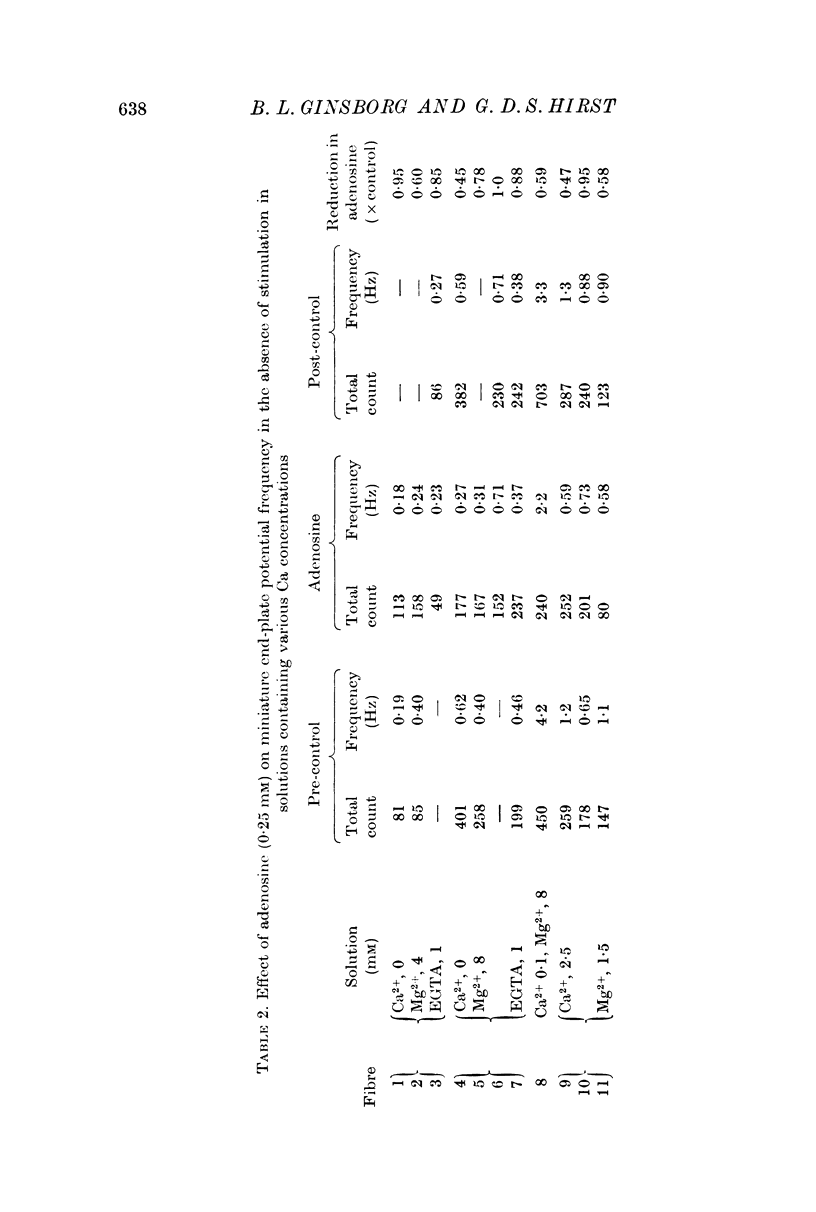
2. Adenosine (0·025-10 mM) reversibly reduced the quantum content of end-plate potentials and the frequency of miniature end-plate potentials to about half the control values in preparations in which transmission was blocked with high Mg2+ and/or low Ca2+ concentrations. Where transmission was blocked with tubocurarine, the amplitude of end-plate potentials was reduced to about half the control values by adenosine.
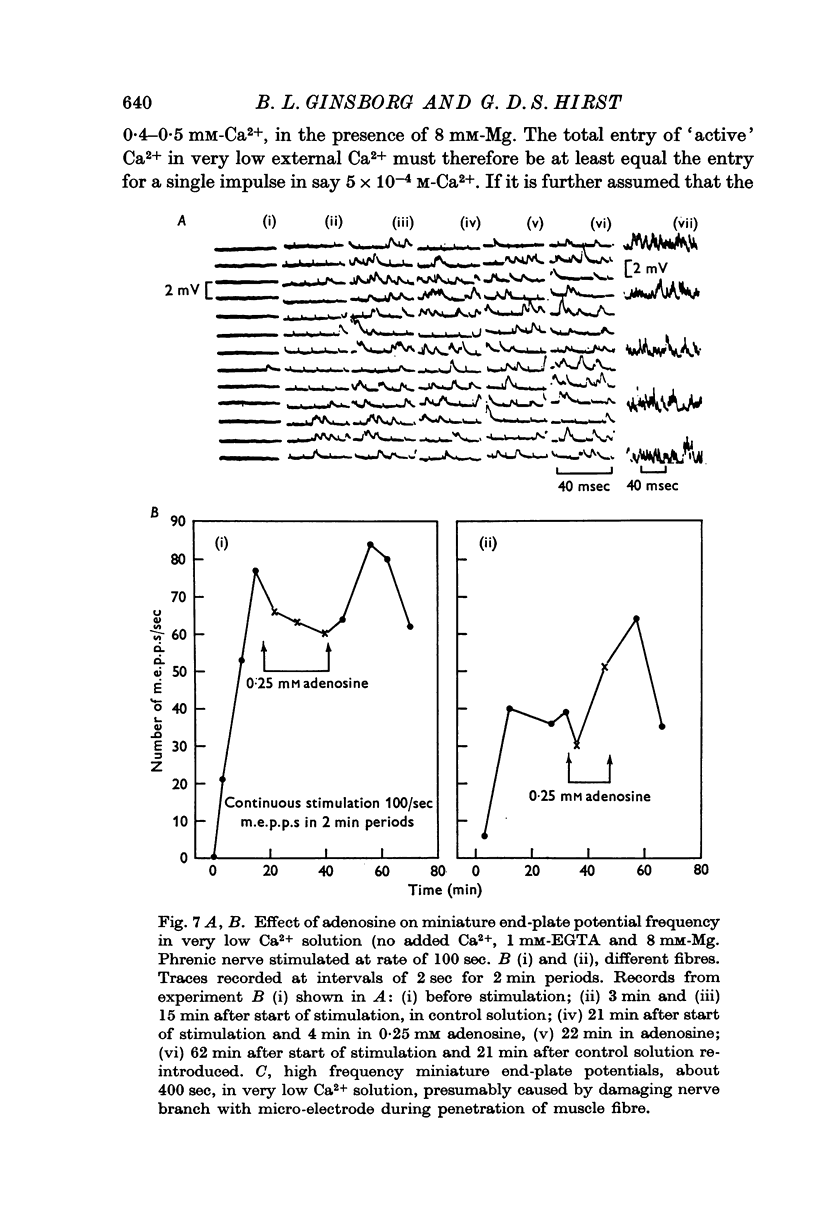
3. In solutions with very low Ca2+ concentrations (no added Ca2+ and 1 mM-EGTA) adenosine had a smaller effect on the frequency of miniature end-plate potentials.
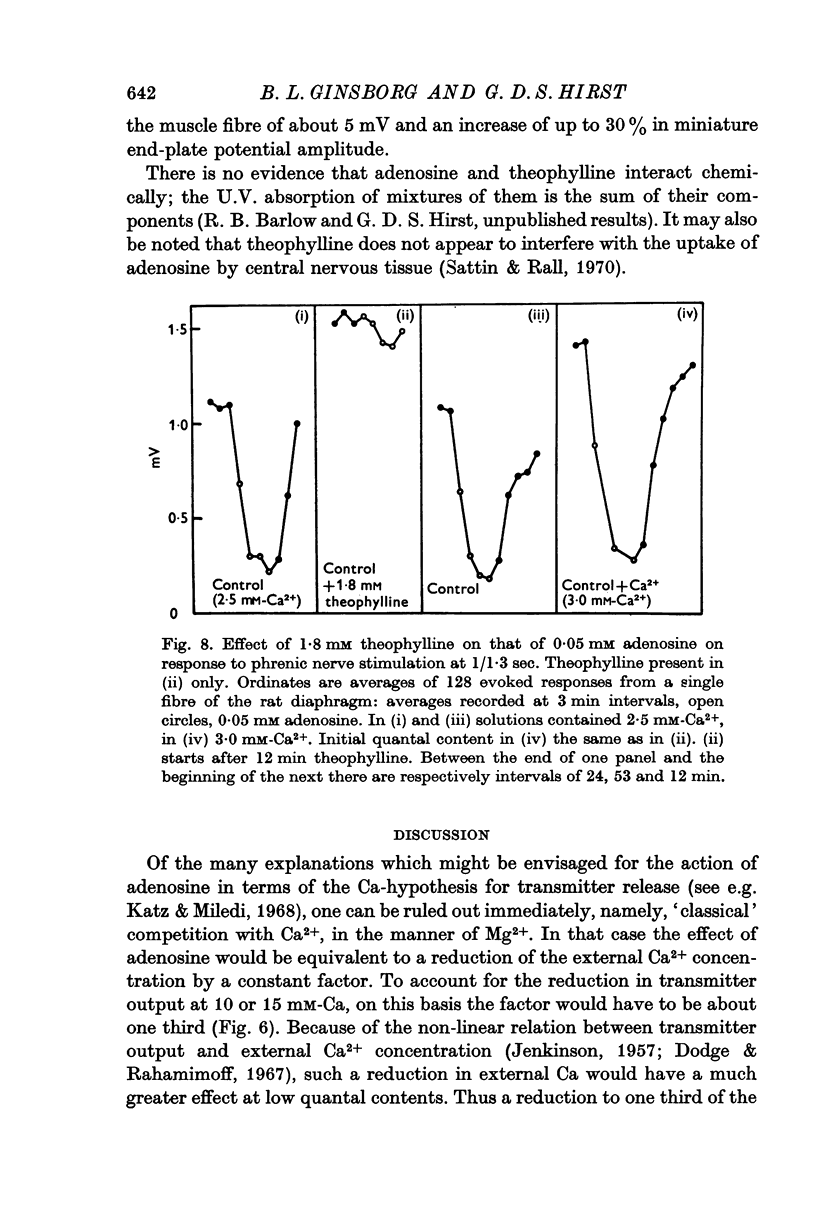
4. The effect of adenosine (0·025 and 0·05 mM) was abolished by theophylline (1·8 mM).
5. The results are discussed in relation to the increase in cyclic AMP in brain slices caused by adenosine and the abolition of this effect by theophylline (Sattin & Rall, 1970).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham L. S., Holt D. A., Sims M. The effect of Ca2+ on the adenyl cyclase of calf brain. Biochim Biophys Acta. 1970 Feb 24;201(2):250–260. doi: 10.1016/0304-4165(70)90299-0. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Niedergerke R. Effects of calcium on the contraction of the hypodynamic frog heart. J Physiol. 1970 Dec;211(2):389–421. doi: 10.1113/jphysiol.1970.sp009284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ D. D., Nishi S. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit. Br J Pharmacol. 1971 Feb;41(2):331–338. doi: 10.1111/j.1476-5381.1971.tb08033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. Cyclic AMP, transmitter release and the effect of adenosine on neuromuscular transmission. Nat New Biol. 1971 Jul 14;232(28):63–64. doi: 10.1038/newbio232063a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Singer J. J. Evidence for a role of cyclic AMP in neuromuscular transmission. Proc Natl Acad Sci U S A. 1969 Sep;64(1):134–141. doi: 10.1073/pnas.64.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968 Aug;197(3):639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Wickelgren W. O., Ber1anek R. Effects of iontophoretically applied drugs on spinal interneurons of the lamprey. J Physiol. 1970 May;207(3):653–665. doi: 10.1113/jphysiol.1970.sp009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee D. A., Schorderet M., Greengard P. Adenosine 3',5'-monophosphate in nervous tissue: increase associated with synaptic transmission. Science. 1971 Mar 19;171(3976):1156–1158. doi: 10.1126/science.171.3976.1156. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. E. Post-tetanic increase in frequency of miniature end-plate potentials in calcium-free solutions. J Physiol. 1967 Sep;192(2):54P–55P. [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]


