Abstract
1. To obtain information about structural events that occur in axons, changes in light scattering from squid giant axons were measured during action potentials and voltage-clamp steps.
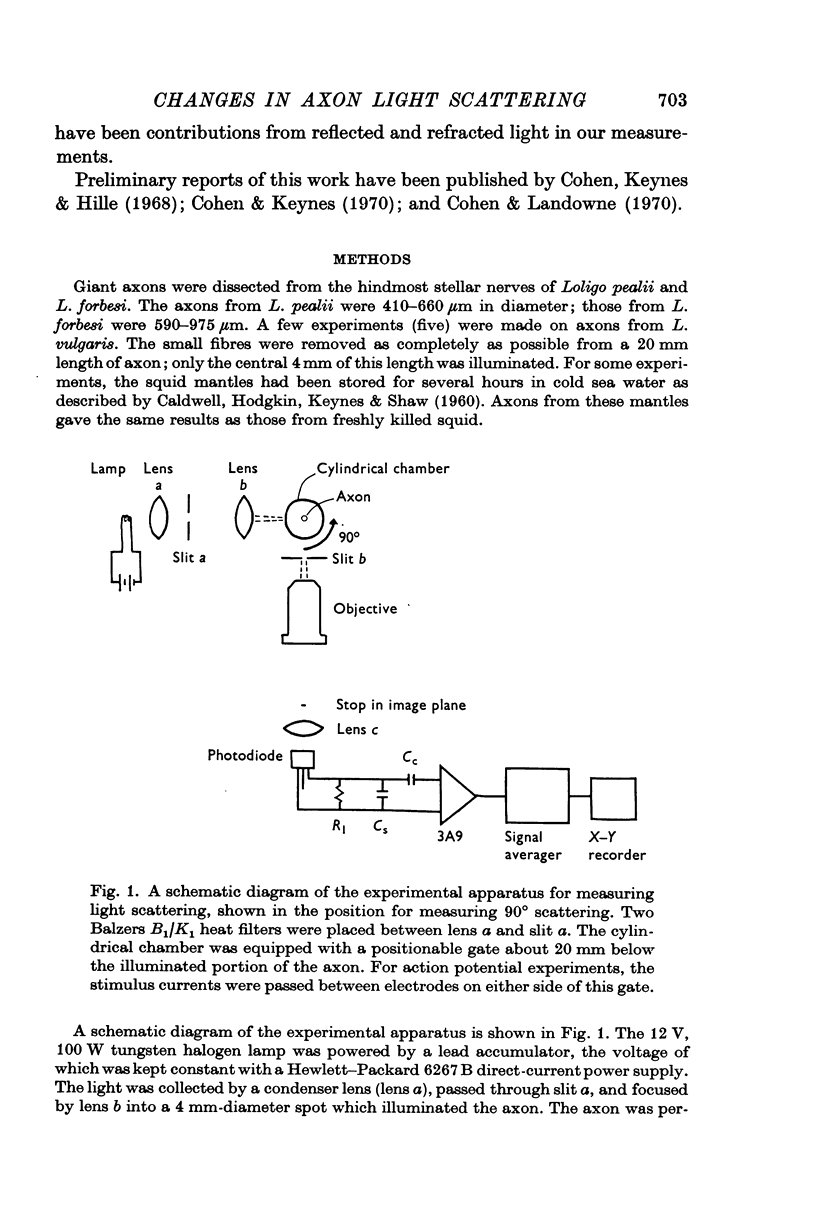
2. The scattering changes were measured at several scattering angles. Because the changes in scattering divided by the resting scattering were between 10-6 and 10-5, signal-averaging techniques were used to increase the signal-to-noise ratio.
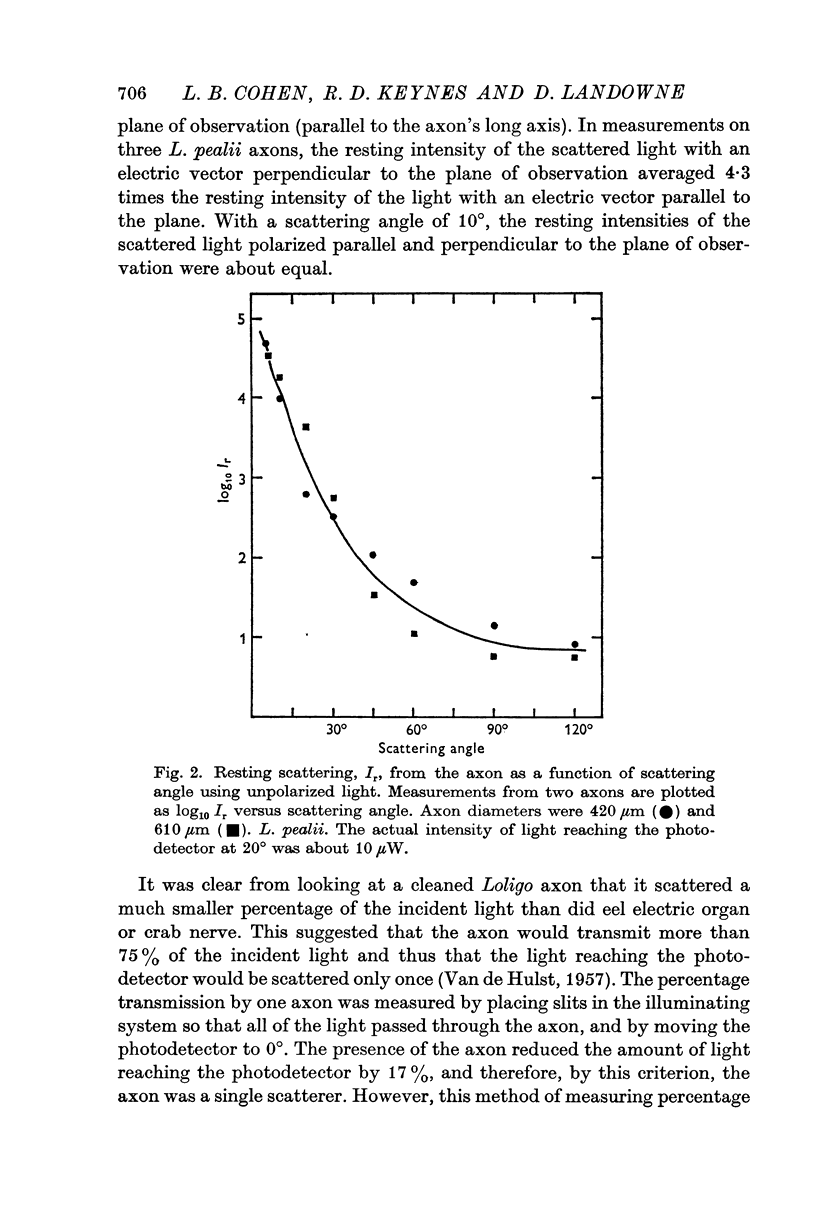
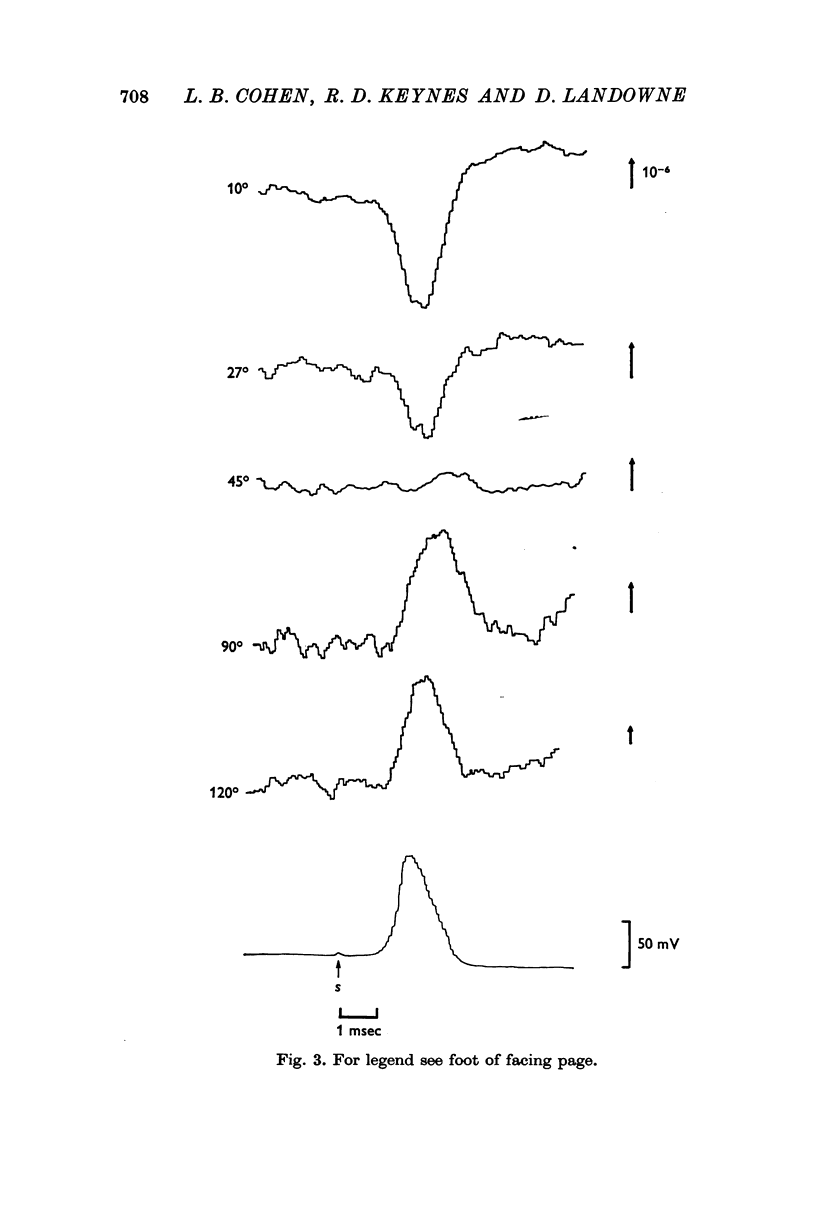
3. The scattering changes during the action potential were different at different angles. Two types were found, one at 10-30° (forward angles) and the other at 60-120° (right angles).
4. At forward angles, there was a transient scattering decrease during the action potential. The time course of the change was similar to that of the action potential; this change was thought to be potential-dependent.
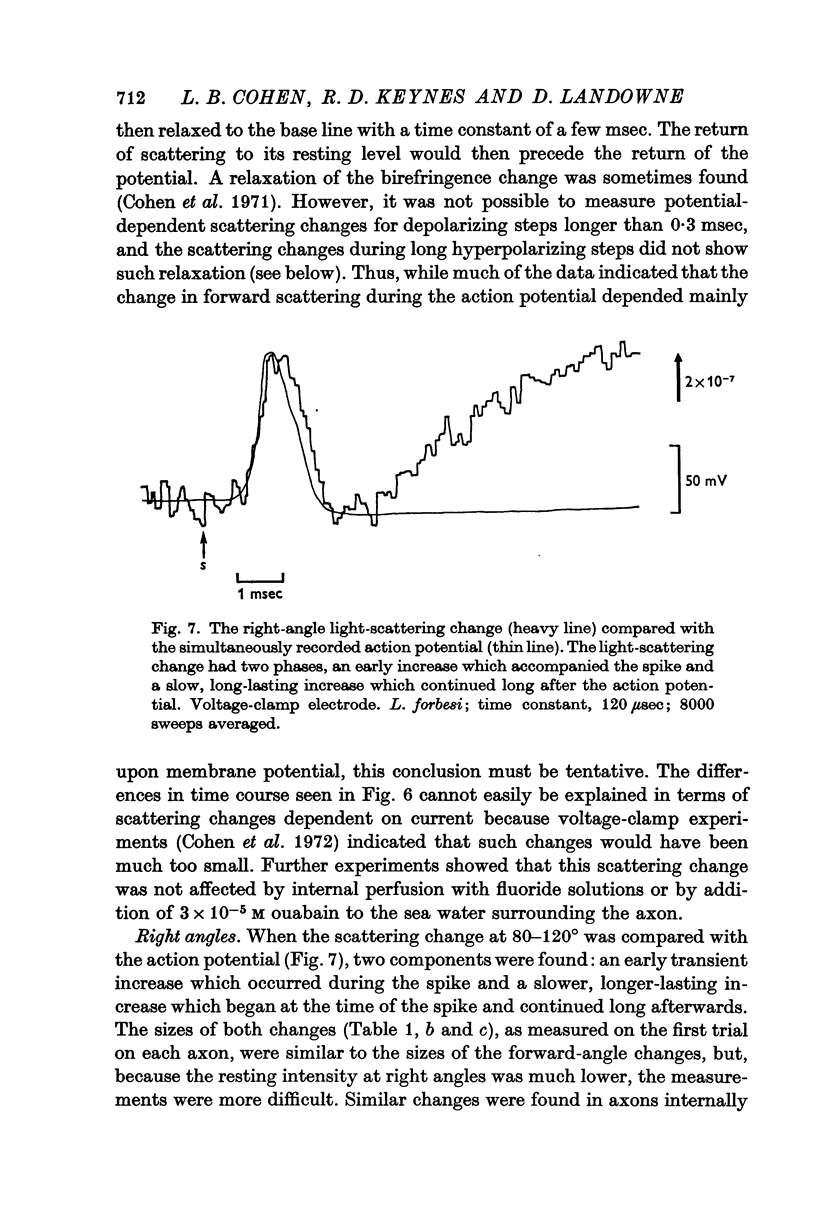
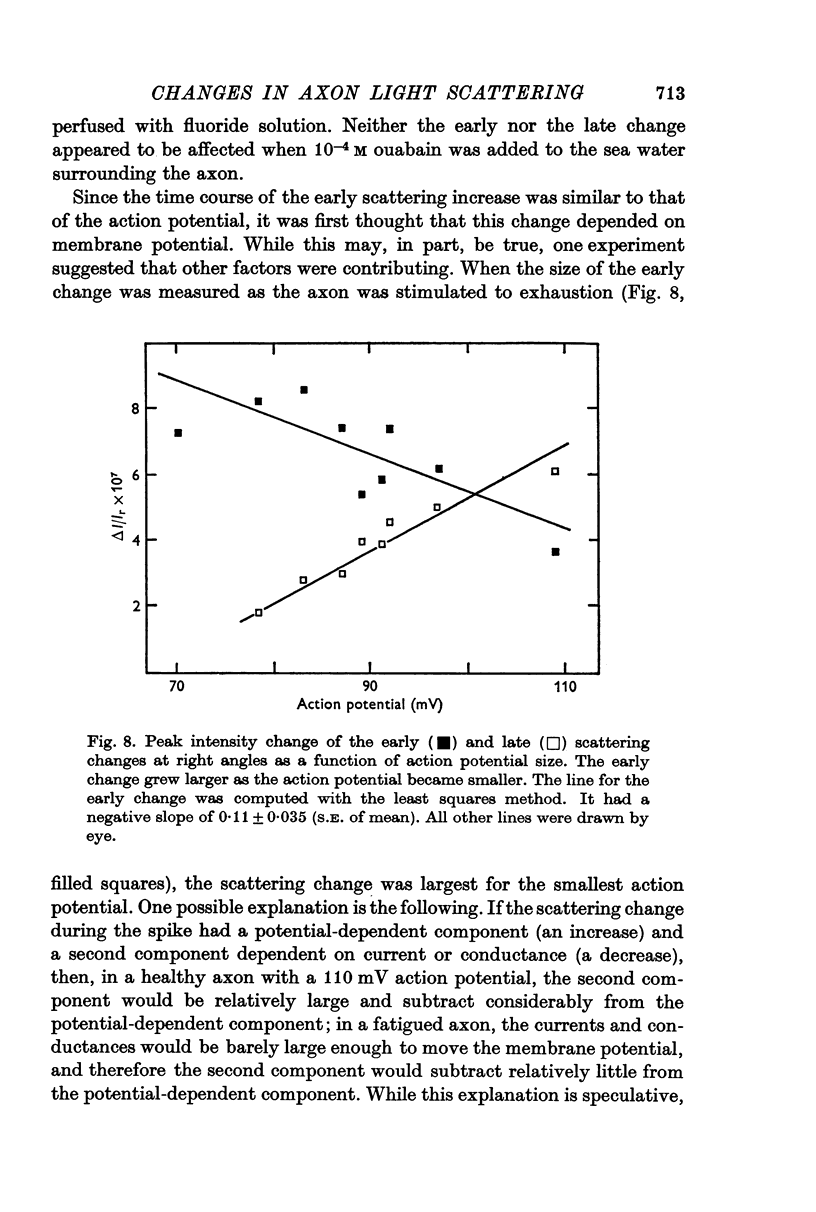
5. At right angles, there was a transient scattering increase during the action potential followed later by a second, longer-lasting increase. Indirect evidence indicated that neither component could be totally potential-dependent.
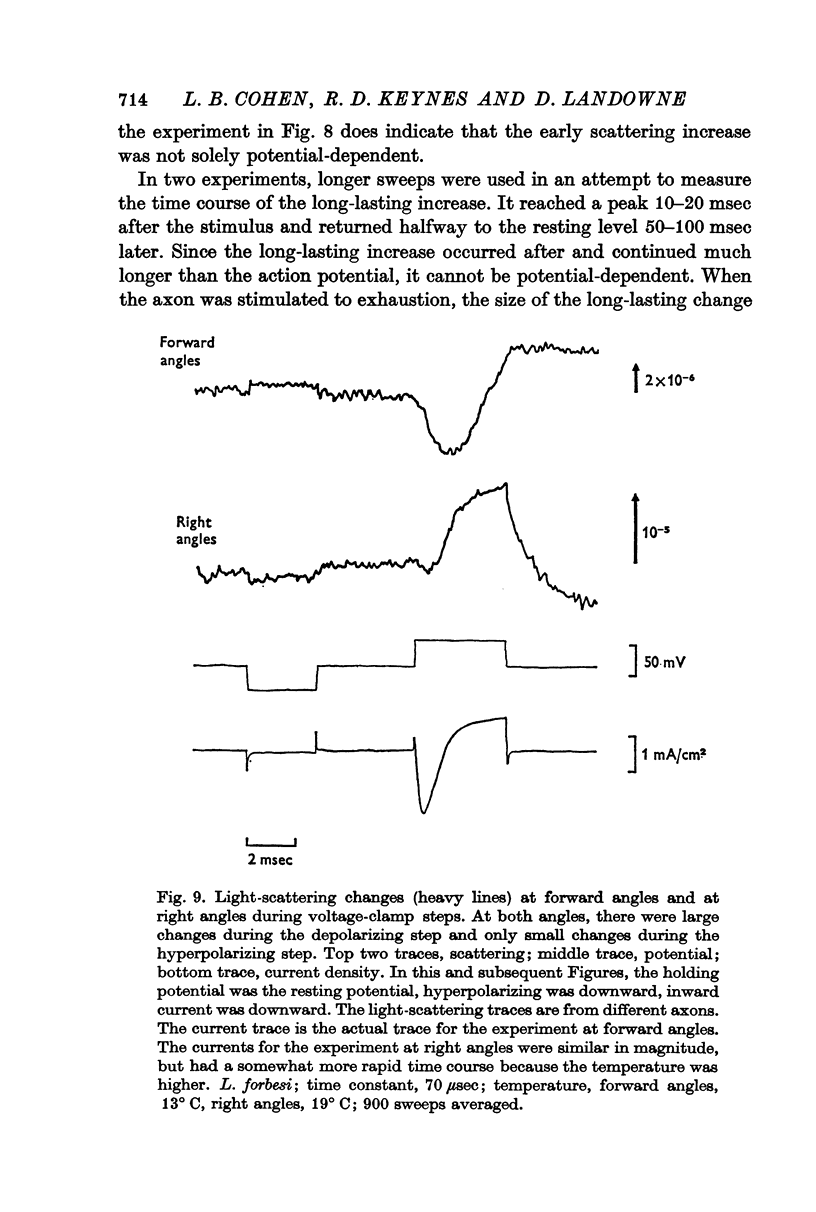
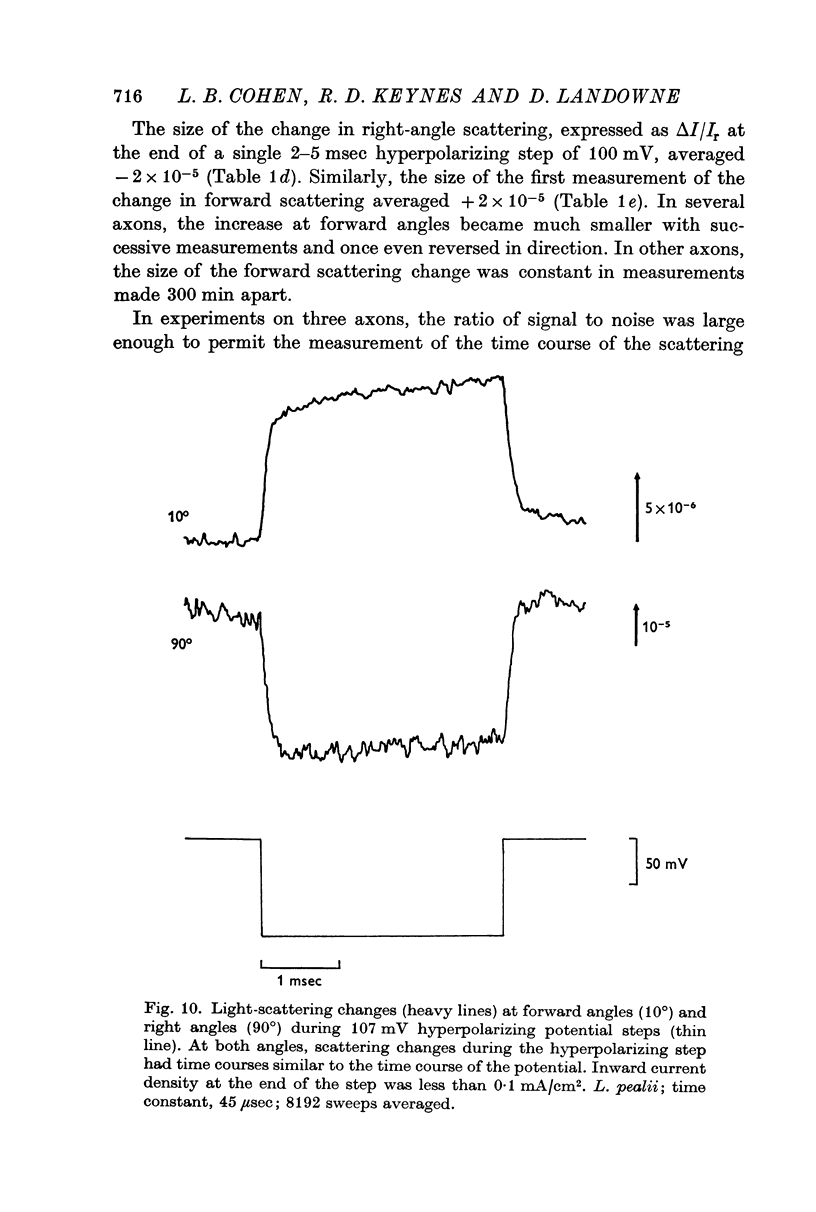
6. To further analyse these effects, scattering was measured during voltage-clamp steps. The changes seen during hyperpolarizing steps were presumed to be potential-dependent; again two different changes were found, one at forward angles and one at right angles.
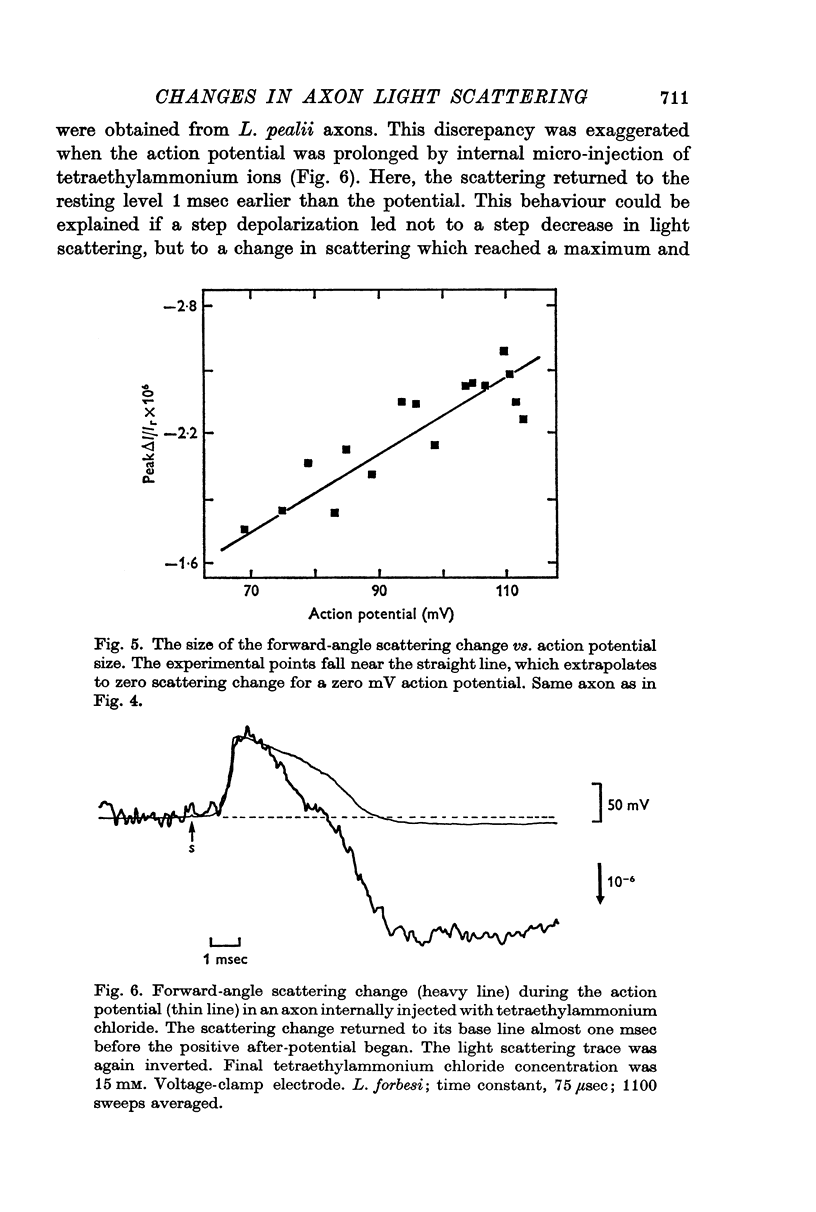
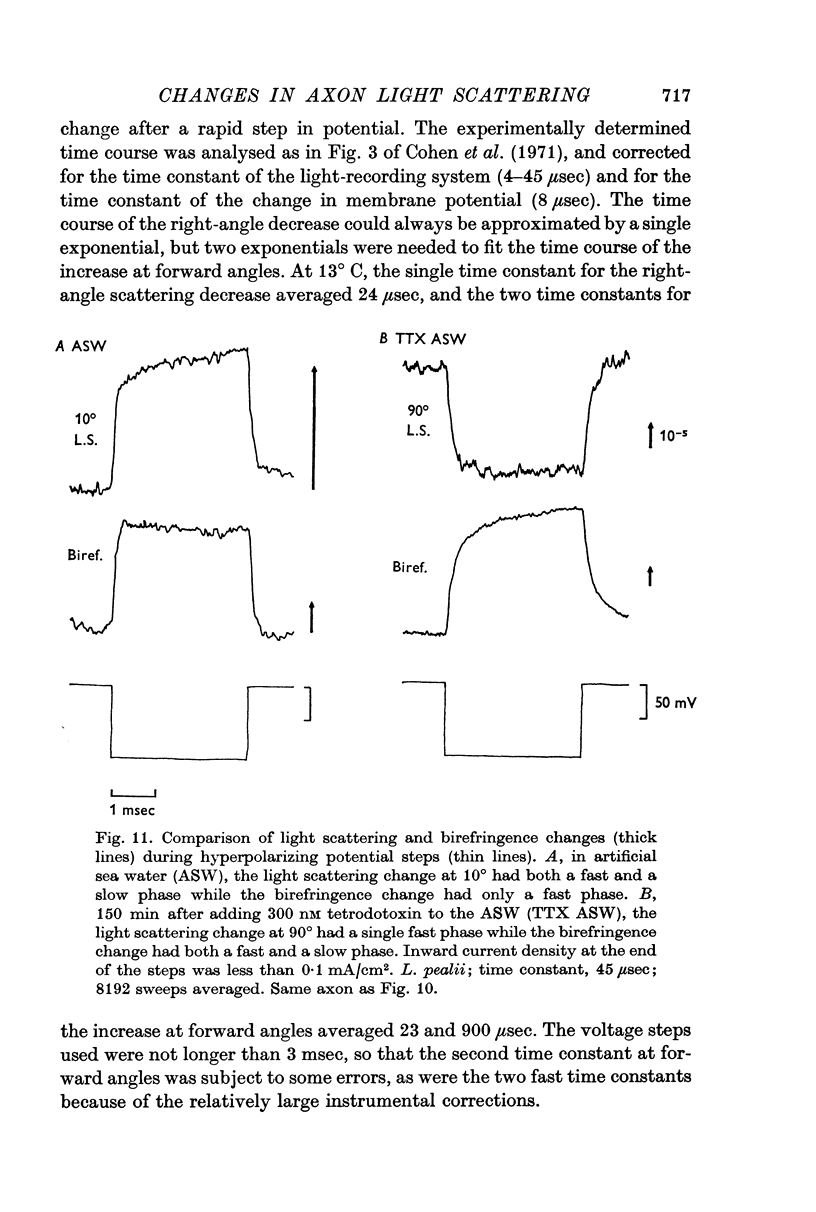
7. The potential-dependent change at right angles occurred with a time course that could be approximated by a single exponential with a time constant τ = 24 μsec. The change at forward angles required two exponentials, τ1 = 23 μsec, τ2 = 900 μsec, to represent its time course.
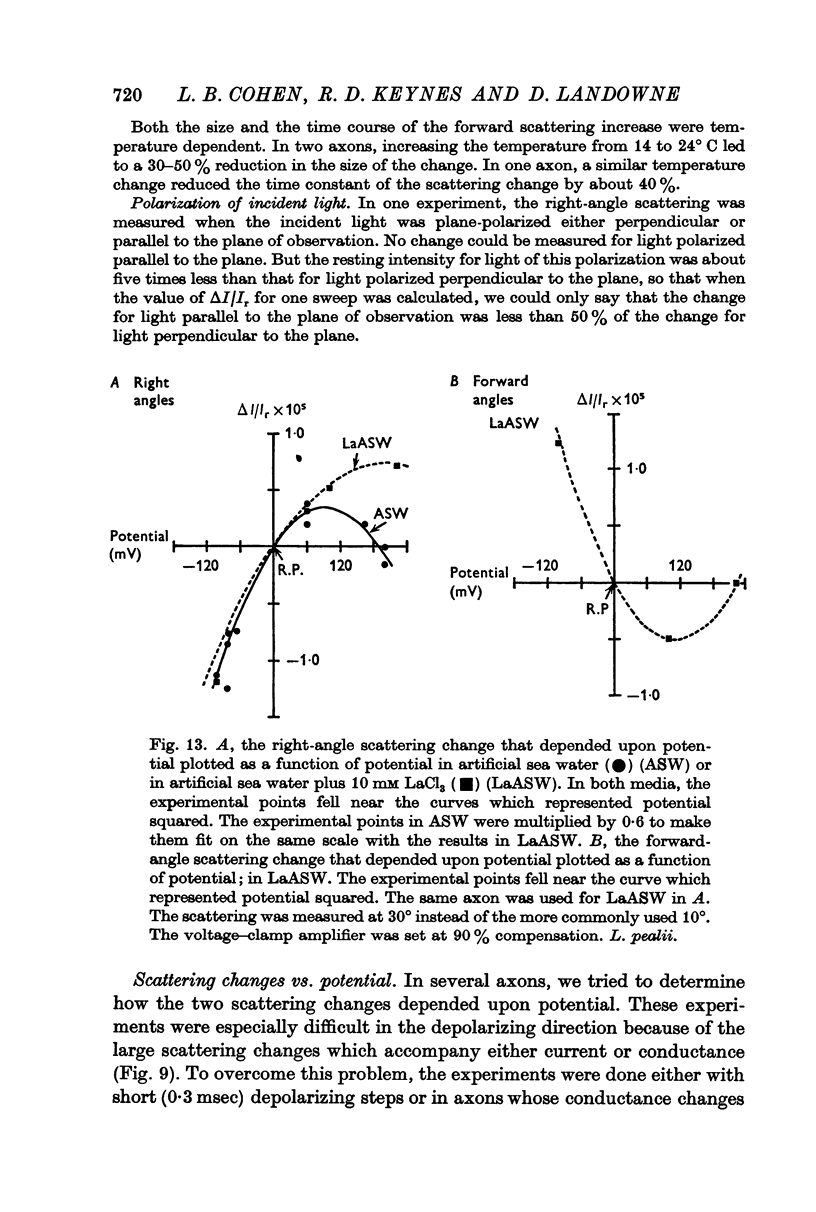
8. The size of both potential-dependent changes was proportional to the square of potential. The change at right angles, but not that at forward angles, was increased in size by the addition of butanol or octanol to the bathing solution.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D., Landowne D., Rojas E. Analysis of the potential-dependent changes in optical retardation in the squid giant axon. J Physiol. 1971 Oct;218(1):205–237. doi: 10.1113/jphysiol.1971.sp009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D. Light scattering and birefringence changes during activity in the electric organ of electrophorus electricus. J Physiol. 1969 Aug;203(2):489–509. doi: 10.1113/jphysiol.1969.sp008876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D. Changes in light scattering associated with the action potential in crab nerves. J Physiol. 1971 Jan;212(1):259–275. doi: 10.1113/jphysiol.1971.sp009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in axon light scattering that accompany the action potential: current-dependent components. J Physiol. 1972 Aug;224(3):727–752. doi: 10.1113/jphysiol.1972.sp009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The effect of stimulation on the opacity of a crustacean nerve trunk and its relation to fibre diameter. J Physiol. 1950 Oct 16;111(3-4):283–303. doi: 10.1113/jphysiol.1950.sp004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Huxley A. F. Resting and action potentials in single nerve fibres. J Physiol. 1945 Oct 15;104(2):176–195. doi: 10.1113/jphysiol.1945.sp004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Bangham A. D. The action of anaesthetics on phospholipid membranes. Biochim Biophys Acta. 1969 Oct 14;193(1):92–104. doi: 10.1016/0005-2736(69)90062-5. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liudkovskaia P. G., Emel'ianov V. B., Lemazhikhin B. K. Issledovanie opticheskikh svoistv gigantskogo aksona kal'mara v pokoe i v raznye fazy vozbuzhdeniia. Tsitologiia. 1965 Jul-Aug;7(4):520–530. [PubMed] [Google Scholar]
- SKOU J. C. Local anaesthetics. VI. Relation between blocking potency and penetration of a monomolecular layer of lipoids from nerves. Acta Pharmacol Toxicol (Copenh) 1954;10(4):325–337. doi: 10.1111/j.1600-0773.1954.tb01349.x. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Relation between the ability of various compounds to block nervous conduction and their penetration into a monomolecular layer of nerve-tissue lipoids. Biochim Biophys Acta. 1958 Dec;30(3):625–629. doi: 10.1016/0006-3002(58)90110-0. [DOI] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Singer I., Takenaka T. Effects of internal and external ionic environment on excitability of squid giant axon. A macromolecular approach. J Gen Physiol. 1965 Jul;48(6):1095–1123. doi: 10.1085/jgp.48.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]


