Abstract
1. Rod-dependent incremental responses were recorded intracellularly in both pigment epithelial cells and horizontal cells of the cat retina. They were elicited by test flashes which were superimposed on background flashes after a delay.
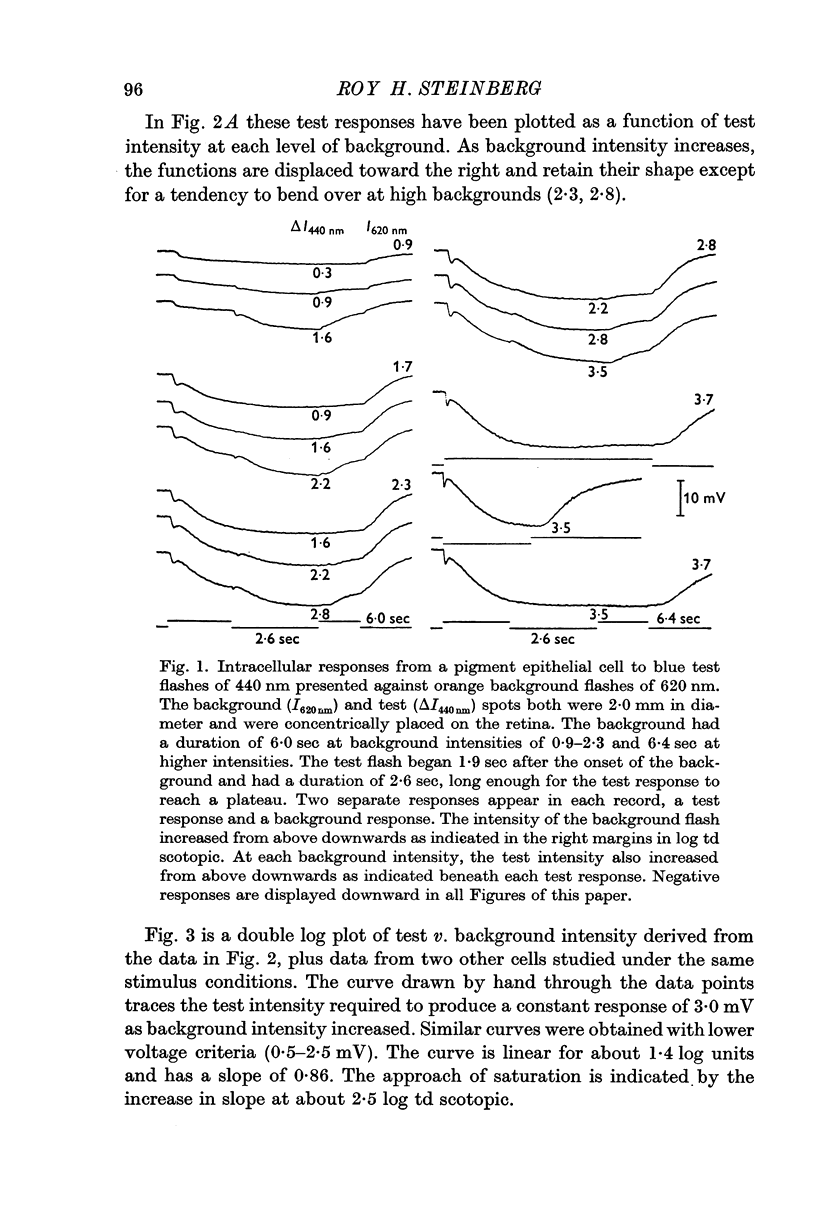
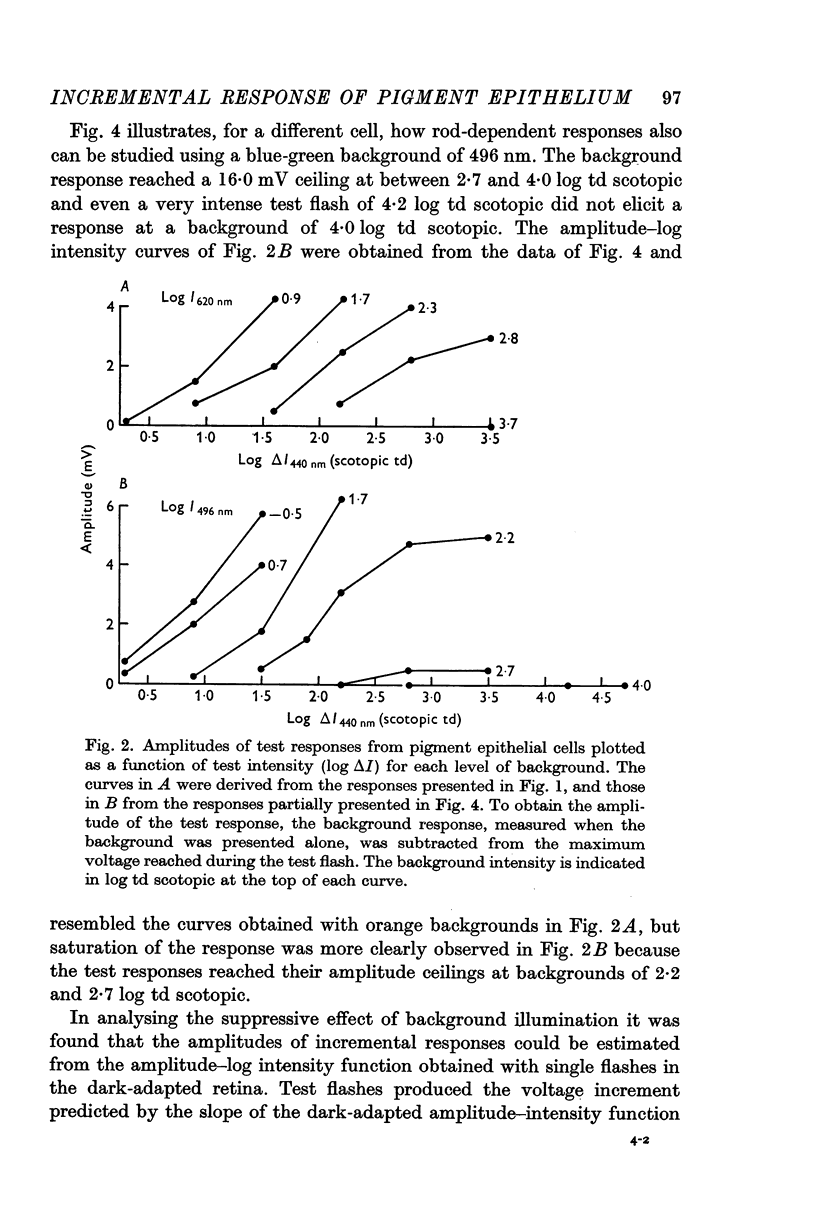
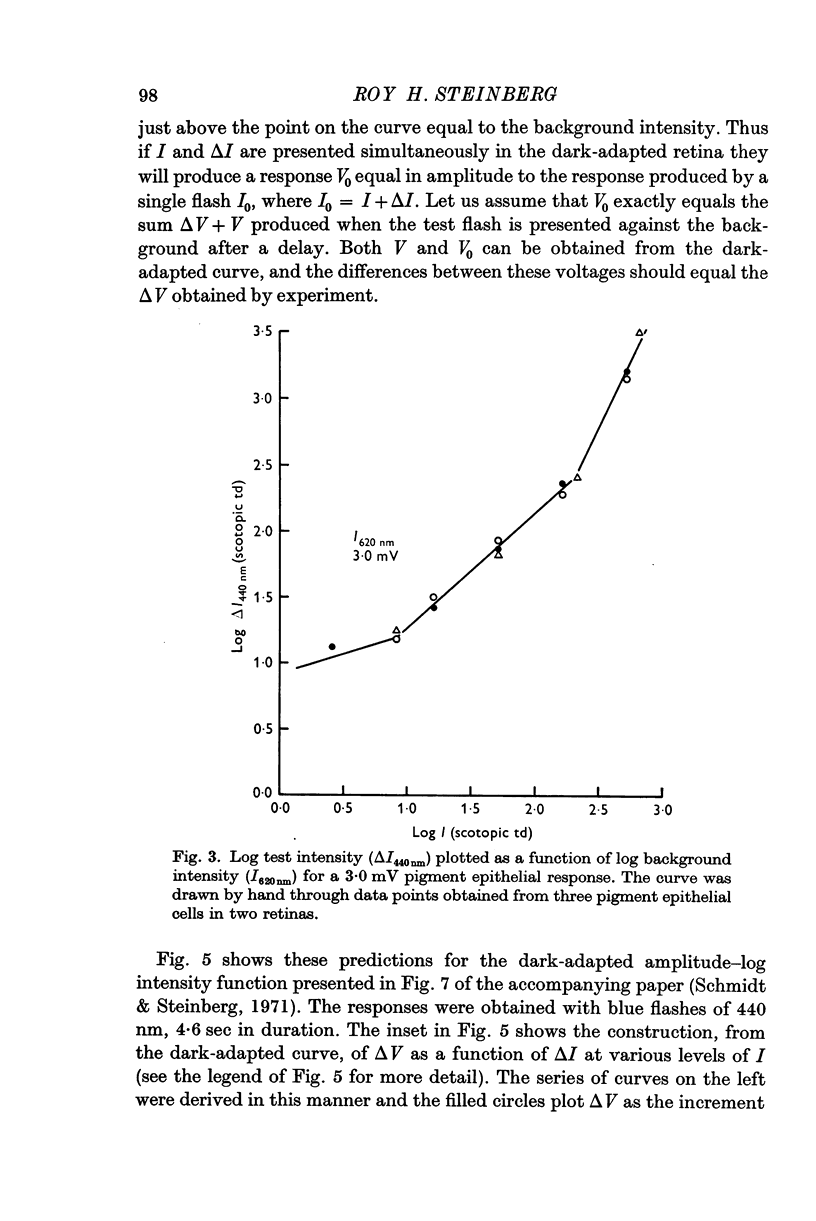
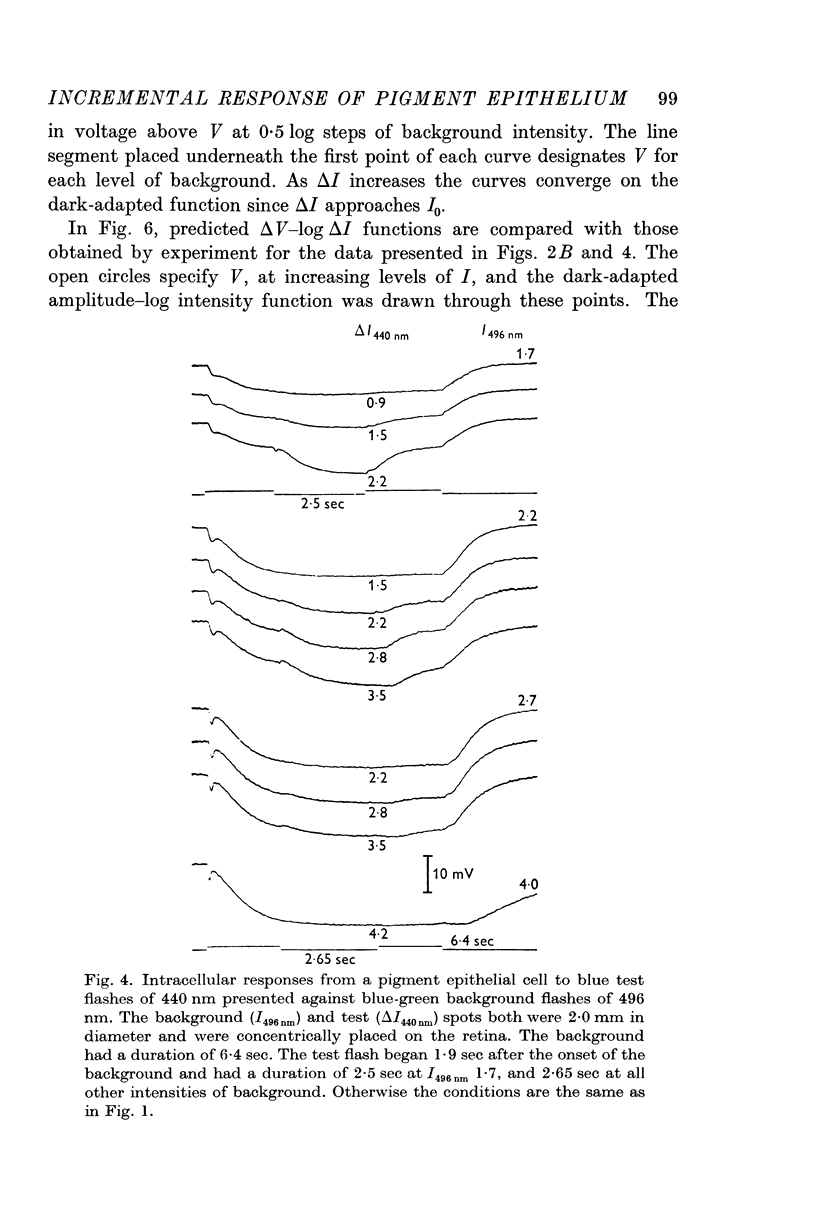
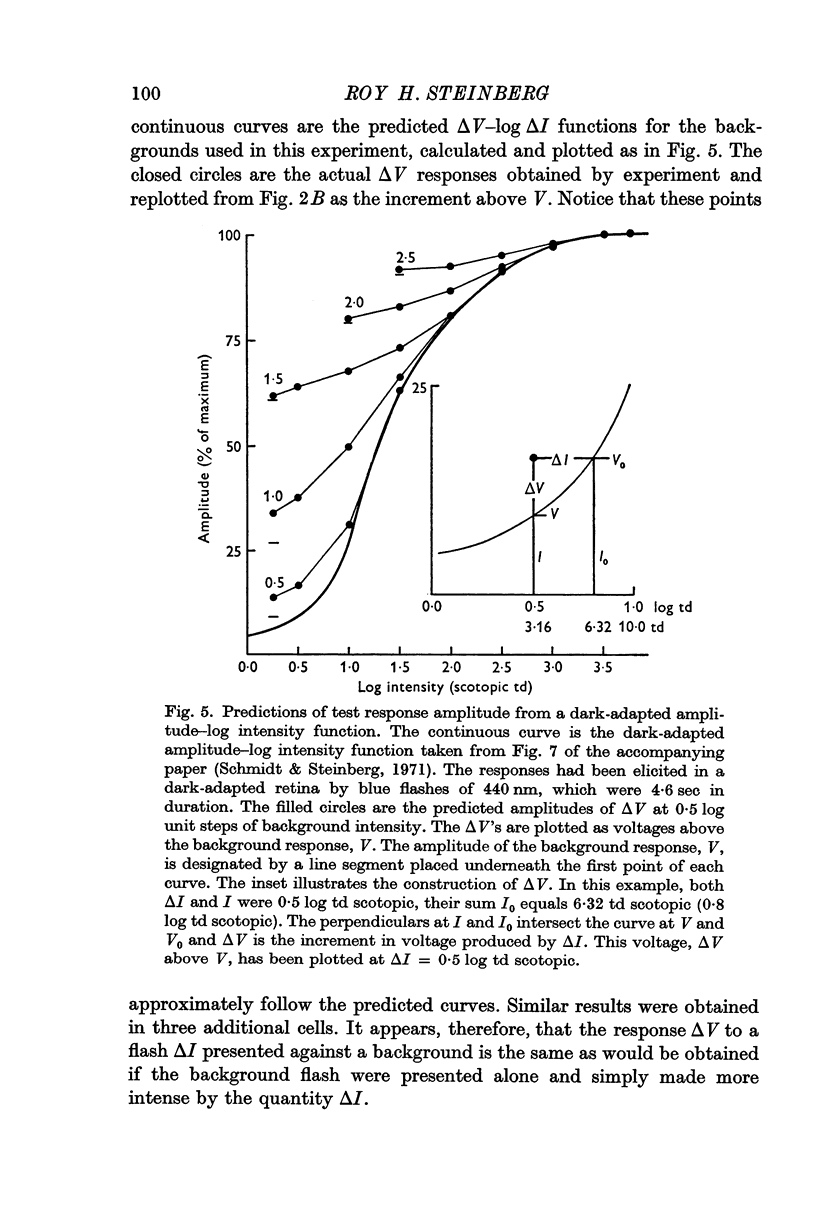
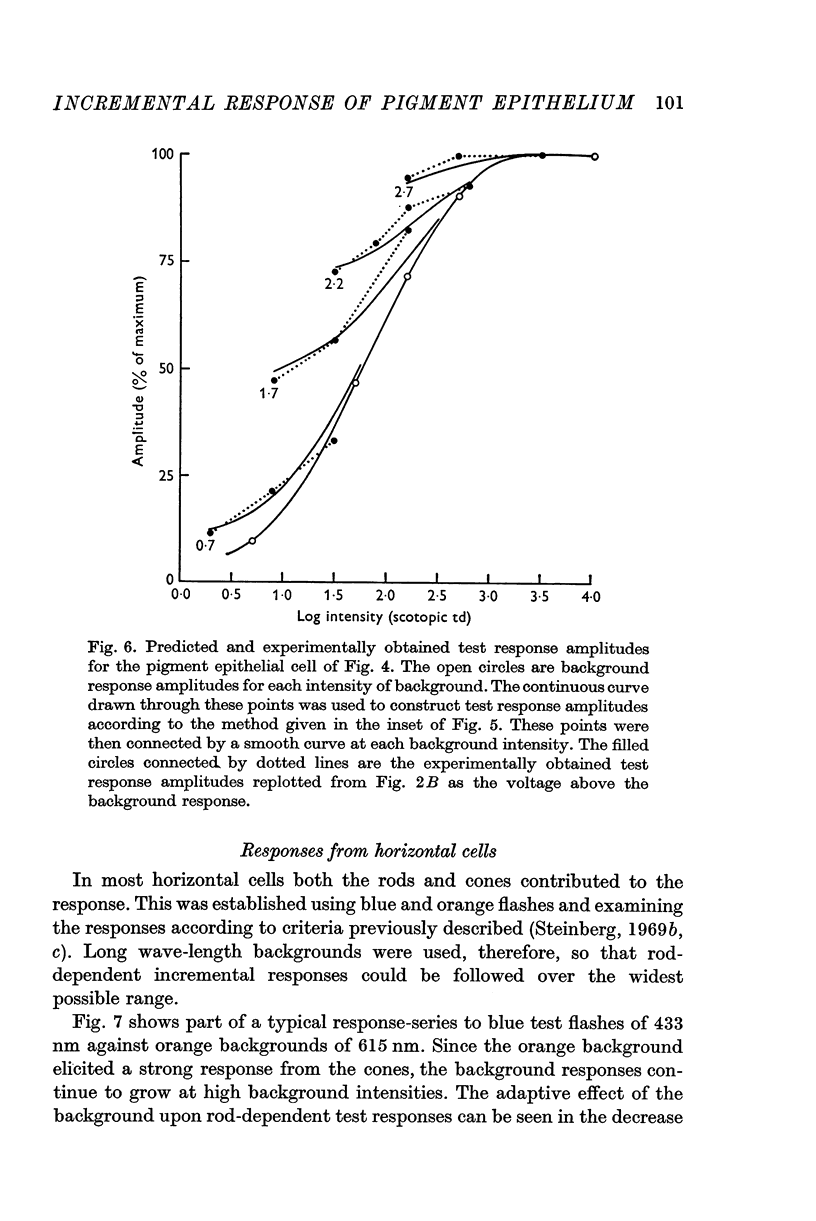
2. In pigment epithelial cells smaller test responses were produced as background intensity was raised. The incremental sensitivity function was linear for about 1·4 log units, with a slope of 0·86, and the approach of saturation occurred at about 2·5 log td scotopic.
3. The amplitude of pigment epithelial test responses could be estimated from the dark-adapted amplitude—log intensity function obtained with single flashes. Test flashes produced the voltage increment predicted by the slope of this function just above the point on the curve equal to the background intensity. The pigment epithelial response to a test flash, therefore, is the response expected if the background were presented alone and made more intense by the amount of the test flash.
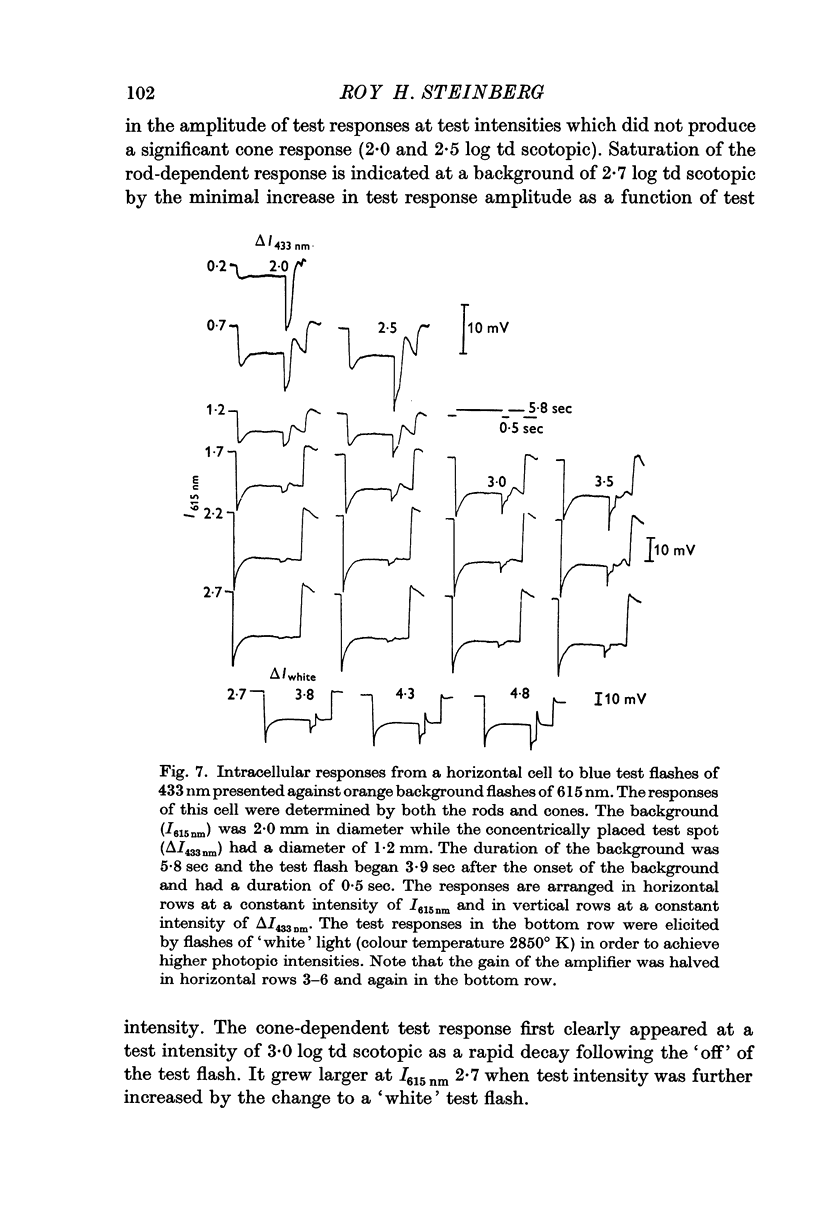
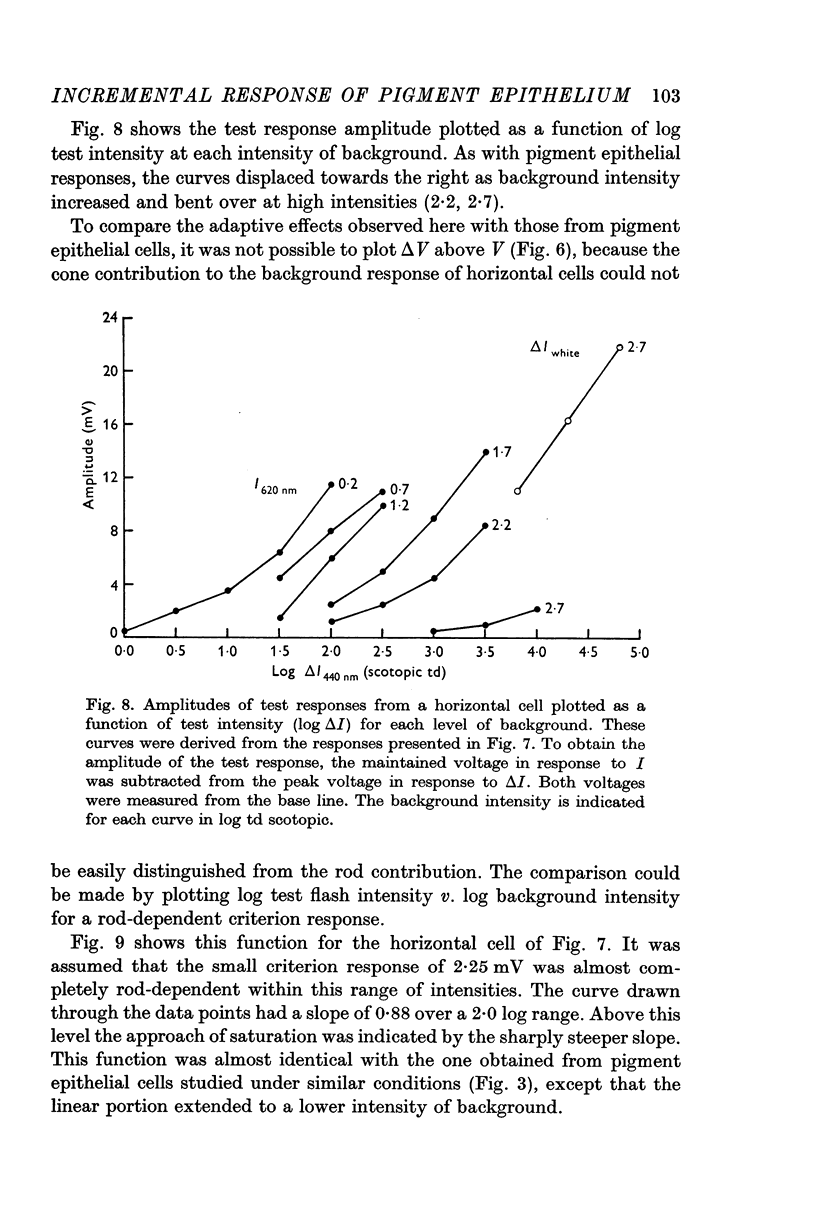
4. Rod-dependent incremental sensitivity functions of horizontal cells closely resembled the ones obtained from pigment epithelial cells.
5. It was concluded that the adaptive effects observed in pigment epithelial cells originated in individual rods. These effects arose from the compressive nature of the dark-adapted amplitude—intensity function. In horizontal cell responses these effects may be modified by the failure of the background response to maintain its initial voltage.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN K. T., WATANABE K. Isolation and identification of a receptor potential from the pure cone fovea of the monkey retina. Nature. 1962 Mar 10;193:958–passim. doi: 10.1038/193958a0. [DOI] [PubMed] [Google Scholar]
- BROWN K. T., WATANABE K. Rod receptor potential from the retina of the night monkey. Nature. 1962 Nov 10;196:547–550. doi: 10.1038/196547a0. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B. Optic nerve impulses and Weber's law. Cold Spring Harb Symp Quant Biol. 1965;30:539–546. doi: 10.1101/sqb.1965.030.01.052. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Brown K. T., Murakami M. Rapid effects of light and dark adaptation upon the receptive field organization of S-potentials and late receptor potentials. Vision Res. 1968 Sep;8(9):1145–1171. doi: 10.1016/0042-6989(68)90024-2. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. J Physiol. 1966 May;184(2):499–510. doi: 10.1113/jphysiol.1966.sp007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Rod and cone independence in the electroretinogram of the dark-adapted monkey's perifovea. J Physiol. 1966 Nov;187(2):455–464. doi: 10.1113/jphysiol.1966.sp008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. Rhodopsin measurement and dark-adaptation in a subject deficient in cone vision. J Physiol. 1961 Apr;156:193–205. doi: 10.1113/jphysiol.1961.sp006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Ford R. W. The cat local electroretinogram to incremental stimuli. Vision Res. 1969 Jan;9(1):1–24. doi: 10.1016/0042-6989(69)90028-5. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O. D. Scotopic and mesopic light adaptation in the cat's retina. Pflugers Arch. 1969;313(2):168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Steinberg R. H. Rod-dependent intracellular responses to light recorded from the pigment epithelium of the cat retina. J Physiol. 1971 Aug;217(1):71–91. doi: 10.1113/jphysiol.1971.sp009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Comparison of the intraretinal b-wave and d.c. component in the area centralis of cat retina. Vision Res. 1969 Mar;9(3):317–331. doi: 10.1016/0042-6989(69)90079-0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. Rod-cone interaction in S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1331–1344. doi: 10.1016/0042-6989(69)90070-4. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Schmidt R., Brown K. T. Intracellular responses to light from cat pigment epithelium: origin of the electroretinogram c-wave. Nature. 1970 Aug 15;227(5259):728–730. doi: 10.1038/227728a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Schmidt R. Identification of horizontal cells as S-potential generators in the cat retina by intracellular dye injection. Vision Res. 1970 Sep;10(9):817–820. doi: 10.1016/0042-6989(70)90160-4. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


