Abstract
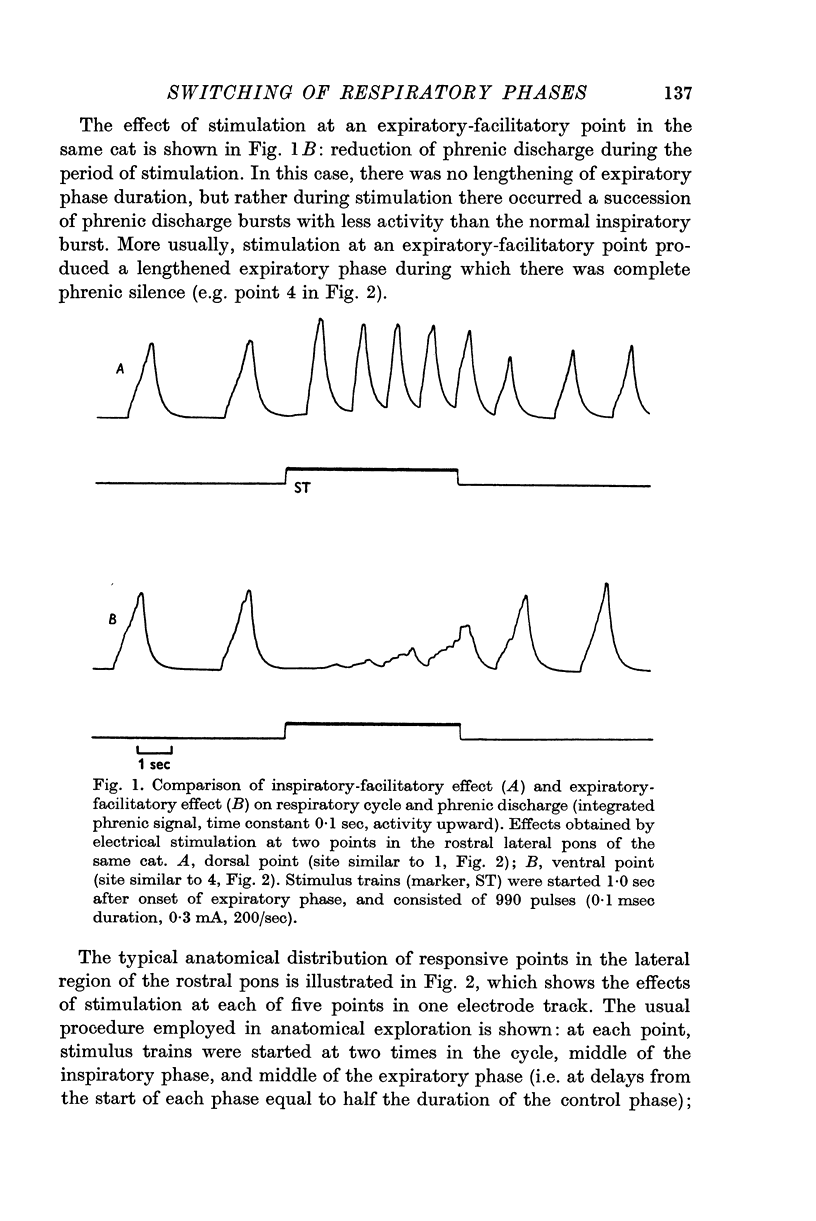
1. In midcollicular-decerebrate, gallamine-paralysed, vagotomized cats, efferent phrenic discharge was recorded as an indicator of the central respiratory cycle. Electrical stimulation (50-250/sec) delivered in the rostral lateral pontine `pneumotaxic centre' region (in and near nucleus parabrachialis), and set to occur at specified times in the cycle, produced powerful respiratory effects: (a) at dorsolateral points, inspiratory-facilitatory effects (increase of phrenic discharge, shortening of the expiratory phase); (b) at ventrolateral points, expiratory-facilitatory effects (decrease of phrenic discharge, shortening of the inspiratory phase, lengthening of the expiratory phase).
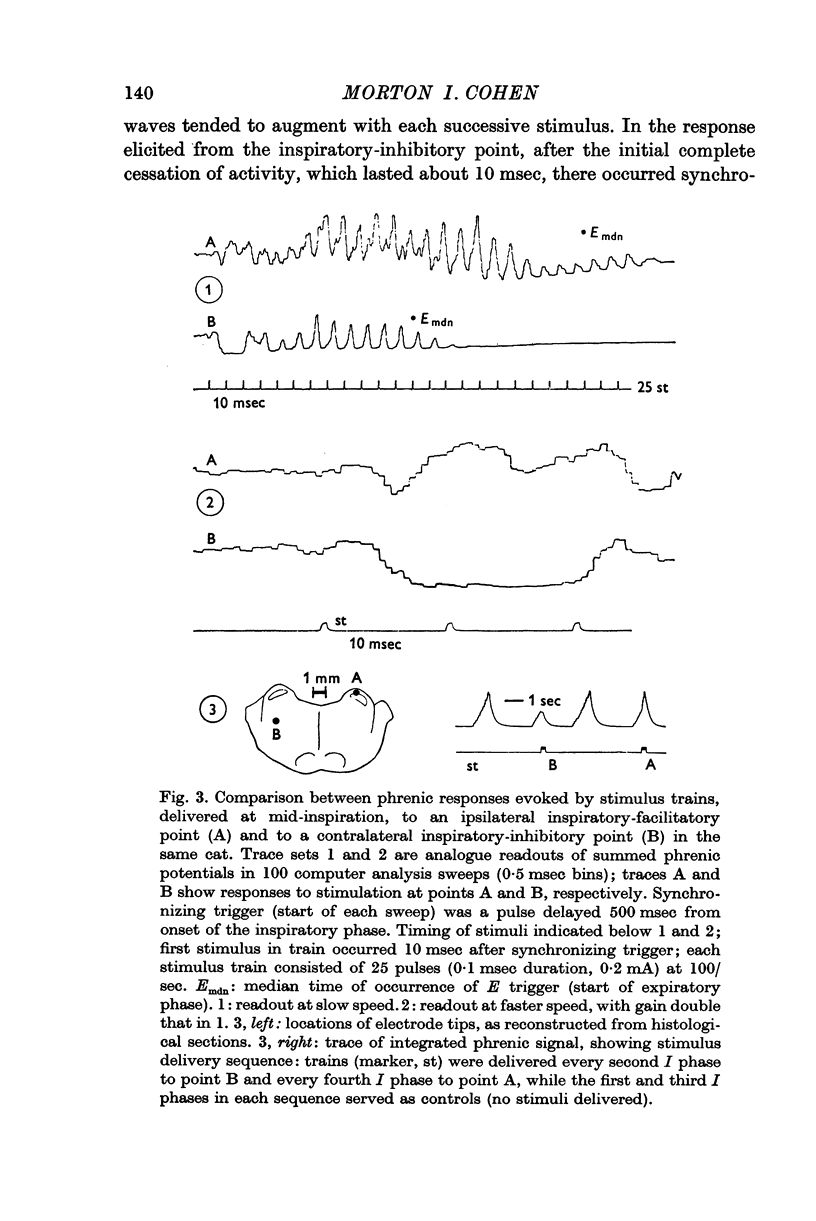
2. At both inspiratory-facilitatory and expiratory-facilitatory points, a single stimulus delivered during the inspiratory phase produced a short-latency (4-7 msec) reduction of phrenic discharge, followed by a wave of increased activity. The short latency of the response indicates the existence of paucisynaptic descending inhibitory pathways. Succeeding stimuli in a high-frequency train produced alternating waves of evoked activity and depression; the form of the responses depended on stimulus frequency and on locus of stimulation.
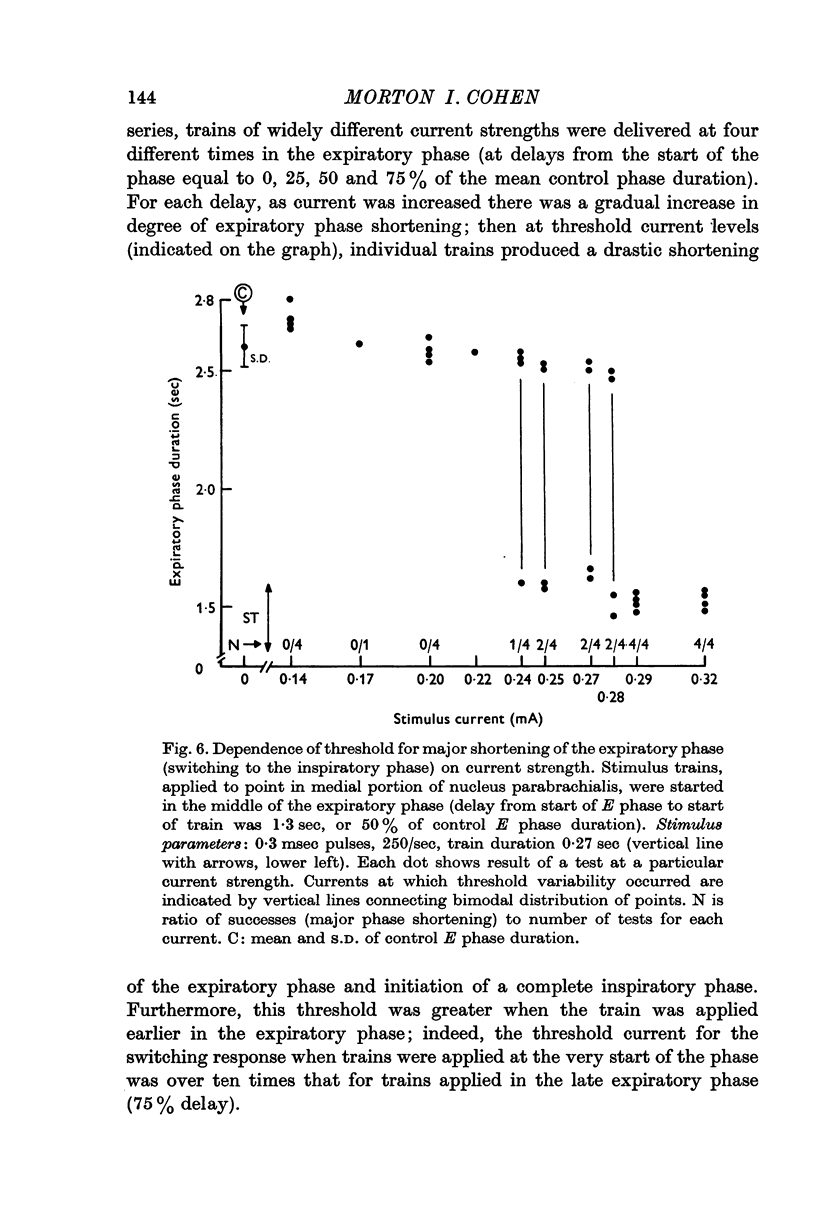
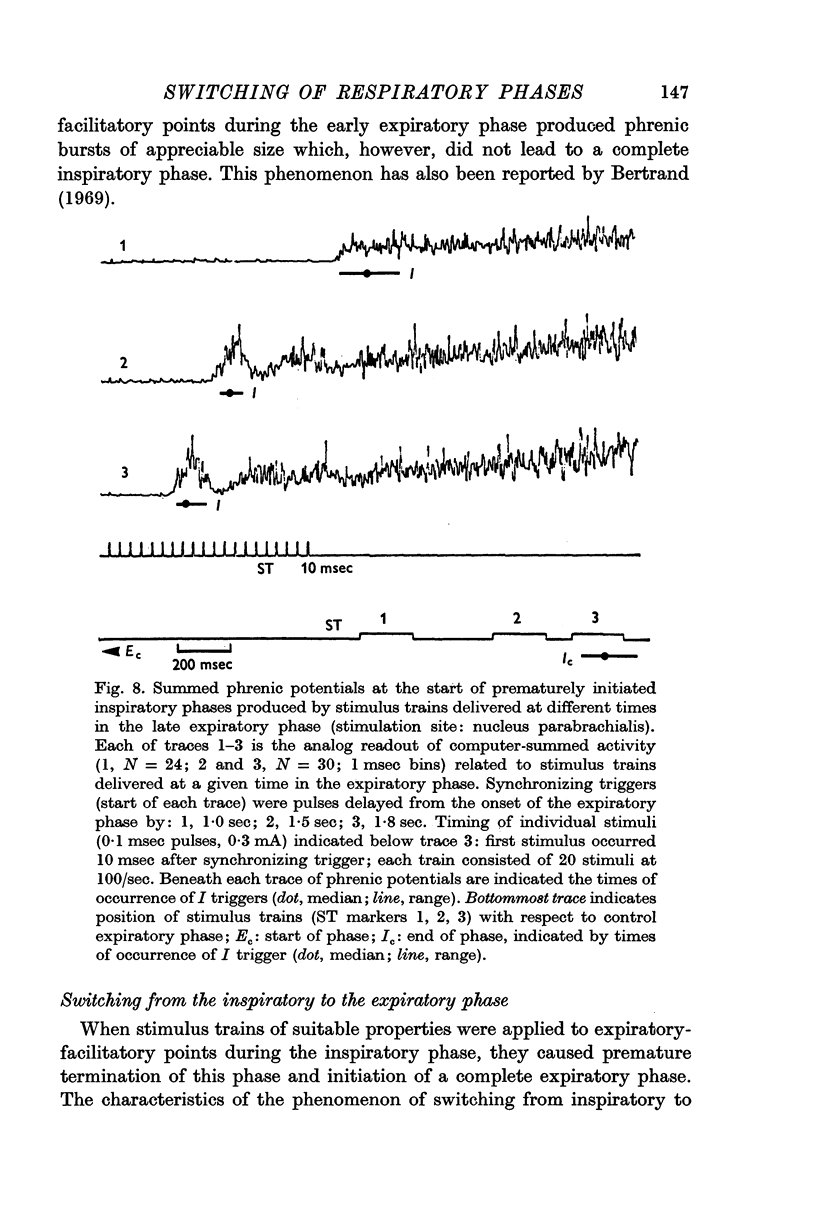
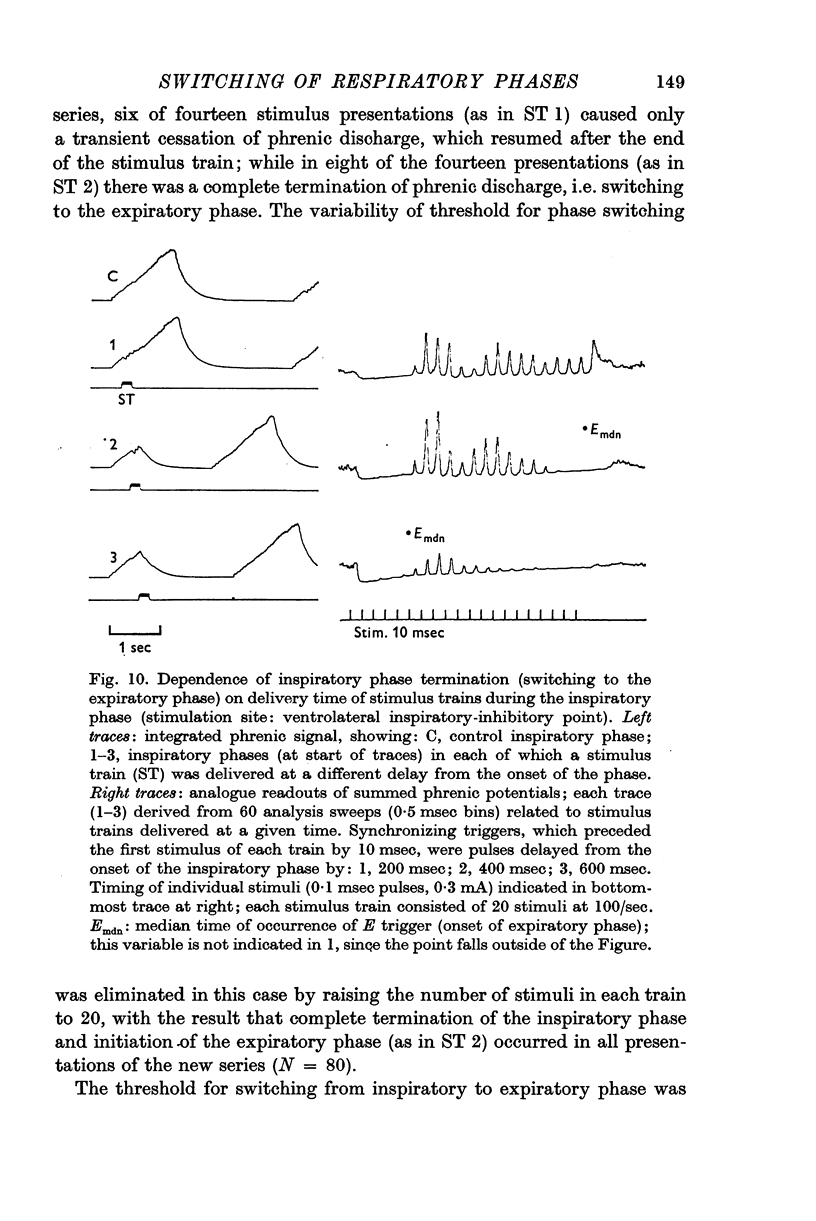
3. At inspiratory-facilitatory points, short stimulus trains (10-30 stimuli) of adequate strength delivered in the middle and late expiratory phase caused early termination of the phase (latency 100-300 msec) and switching to a complete inspiratory phase, in which the phrenic discharge pattern resembled that in a normal inspiratory phase. Similarly, adequate stimulus trains applied at expiratory-facilitatory points during the middle and late inspiratory phase caused early termination of the phase and switching to a complete expiratory phase.
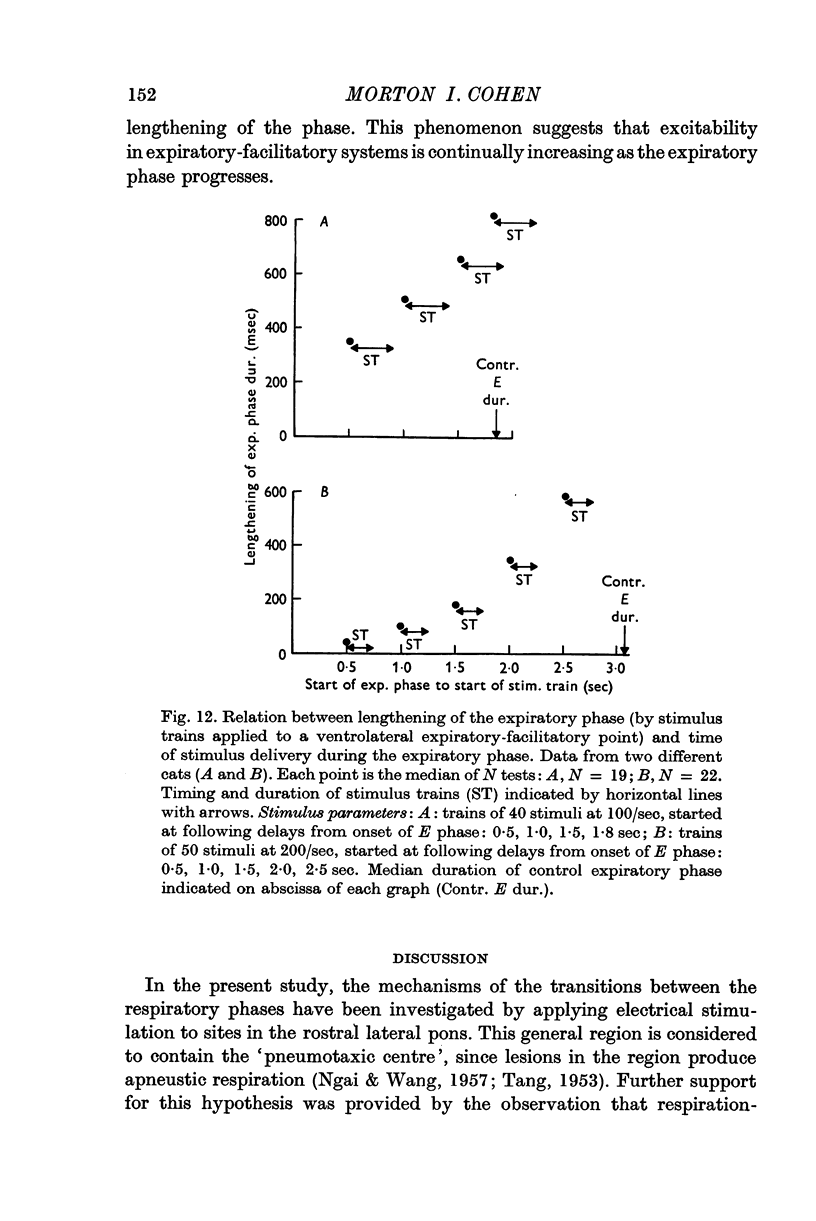
4. The threshold for occurrence of each type of phase-switching response depended on stimulus current, frequency, number of stimuli, and time of stimulus delivery. As stimulus trains were delivered later in the phase, the threshold for switching to the succeeding phase was progressively reduced. Moreover, the nature of the evoked effects was a non-linear function of stimulus characteristics: a small increase of stimulus efficacy changed the system's response from (a) moderate shortening of the phase or transient change in phrenic discharge, to (b) complete termination of the phase.
5. These results indicate that, as each respiratory phase progresses, there is a steady increase of excitability in systems which promote the onset of the succeeding phase. Further, the existence of a relatively sharp threshold for switching of the respiratory phases suggests that the phase transitions occur when critical levels of excitation and inhibition are reached synchronously in populations of respiratory neurones.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Sears T. A. Medullary activation of intercostal fusimotor and alpha motoneurones. J Physiol. 1970 Aug;209(3):739–755. doi: 10.1113/jphysiol.1970.sp009189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER D. W., OLSZEWSKI J. Respiratory responses evoked by electrical stimulation of pons and mesencephalon. J Neurophysiol. 1955 May;18(3):276–287. doi: 10.1152/jn.1955.18.3.276. [DOI] [PubMed] [Google Scholar]
- Bertrand F., Hugelin A. Activité unitaire tonique à modulation périodique respiratoire dans la région du nucleus parabrachialis du chat. J Physiol (Paris) 1968;60 (Suppl 2):402–402. [PubMed] [Google Scholar]
- Bertrand F., Hugelin A. Respiratory synchronizing function of nucleus parabrachialis medialis: pneumotaxic mechanisms. J Neurophysiol. 1971 Mar;34(2):189–207. doi: 10.1152/jn.1971.34.2.189. [DOI] [PubMed] [Google Scholar]
- Bertrand F. Réponse pontophrénique évoquée par stimulation du noyau parabrachialis chez le chat. J Physiol (Paris) 1969;61 (Suppl 2)(Suppl 2 Suppl):220–220. [PubMed] [Google Scholar]
- COHEN M. I. RESPIRATORY PERIODICITY IN THE PARALYZED, VAGOTOMIZED CAT: HYPOCAPNIC POLYPNEA. Am J Physiol. 1964 Apr;206:845–854. doi: 10.1152/ajplegacy.1964.206.4.845. [DOI] [PubMed] [Google Scholar]
- COHEN M. I., WANG S. C. Respiratory neuronal activity in pons of cat. J Neurophysiol. 1959 Jan;22(1):33–50. doi: 10.1152/jn.1959.22.1.33. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons during Hering-Breuer reflex evoked by lung inflation. J Neurophysiol. 1969 May;32(3):356–374. doi: 10.1152/jn.1969.32.3.356. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol. 1968 Mar;31(2):142–165. doi: 10.1152/jn.1968.31.2.142. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Gootman P. M. Spontaneous and evoked oscillations in respiratory and sympathetic discharge. Brain Res. 1969 Nov;16(1):265–268. [PubMed] [Google Scholar]
- Cohen M. I., Hugelin A. Suprapontine reticular control of intrinsic respiratory mechanisms. Arch Ital Biol. 1965 Jun 10;103(2):317–334. [PubMed] [Google Scholar]
- FLOERSHEIM G. L. [On the electromyographic characteristics of respiratory activity]. Helv Physiol Pharmacol Acta. 1960;18:17–24. [PubMed] [Google Scholar]
- HUGELIN A., COHEN M. I. The reticular activating system and respiratory regulation in the cat. Ann N Y Acad Sci. 1963 Jun 24;109:586–603. doi: 10.1111/j.1749-6632.1963.tb13489.x. [DOI] [PubMed] [Google Scholar]
- LILLY J. C., HUGHES J. R., ALVORD E. C., Jr, GALKIN T. W. Brief, noninjurious electric waveform for stimulation of the brain. Science. 1955 Apr 1;121(3144):468–469. doi: 10.1126/science.121.3144.468. [DOI] [PubMed] [Google Scholar]
- NGAI S. H., WANG S. C. Organization of central respiratory mechanisms in the brain stem of the cat: localization by stimulation and destruction. Am J Physiol. 1957 Aug;190(2):343–349. doi: 10.1152/ajplegacy.1957.190.2.343. [DOI] [PubMed] [Google Scholar]
- Polz P., Hunsperger R. W. Reizphysiologischer Nachweis inspiratorisch und exspiratorisch wirksamer Substrate im Ponsabschnitt des Hirnstamms beim Kaninchen. Ein Beitrag zur Frage des pneumotaktischen Mechanismus. Helv Physiol Pharmacol Acta. 1968;26(1):119–135. [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABER E. The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of cat. J Comp Neurol. 1961 Feb;116:27–69. doi: 10.1002/cne.901160104. [DOI] [PubMed] [Google Scholar]
- TANG P. C. Localization of the pneumotaxic center in the cat. Am J Physiol. 1953 Mar;172(3):645–652. doi: 10.1152/ajplegacy.1953.172.3.645. [DOI] [PubMed] [Google Scholar]
- Tan E. S. Brain-stem regions for stimulus-bound and stimulus-related respiration. Exp Neurol. 1967 Apr;17(4):517–528. doi: 10.1016/0014-4886(67)90136-7. [DOI] [PubMed] [Google Scholar]


