Abstract
The membrane properties of the longitudinal smooth muscle of the guinea-pig portal vein were investigated under various experimental conditions.
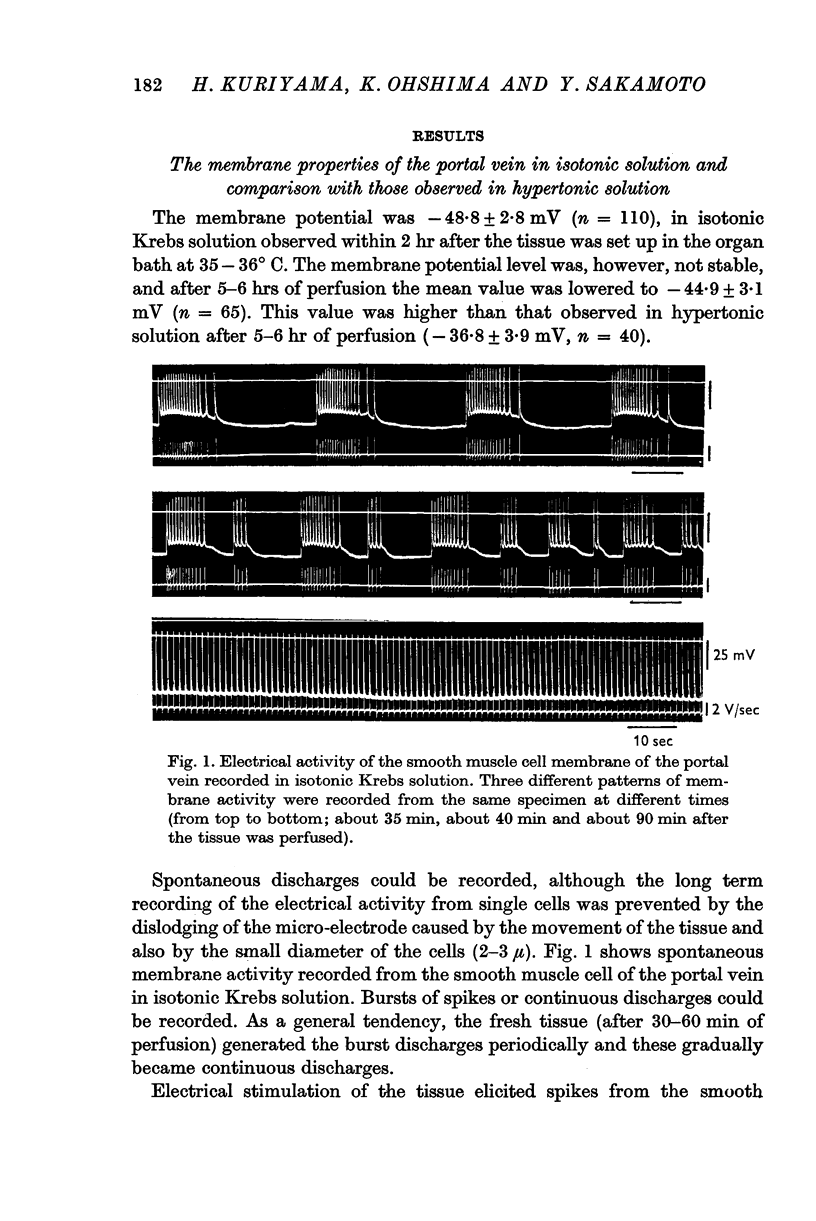
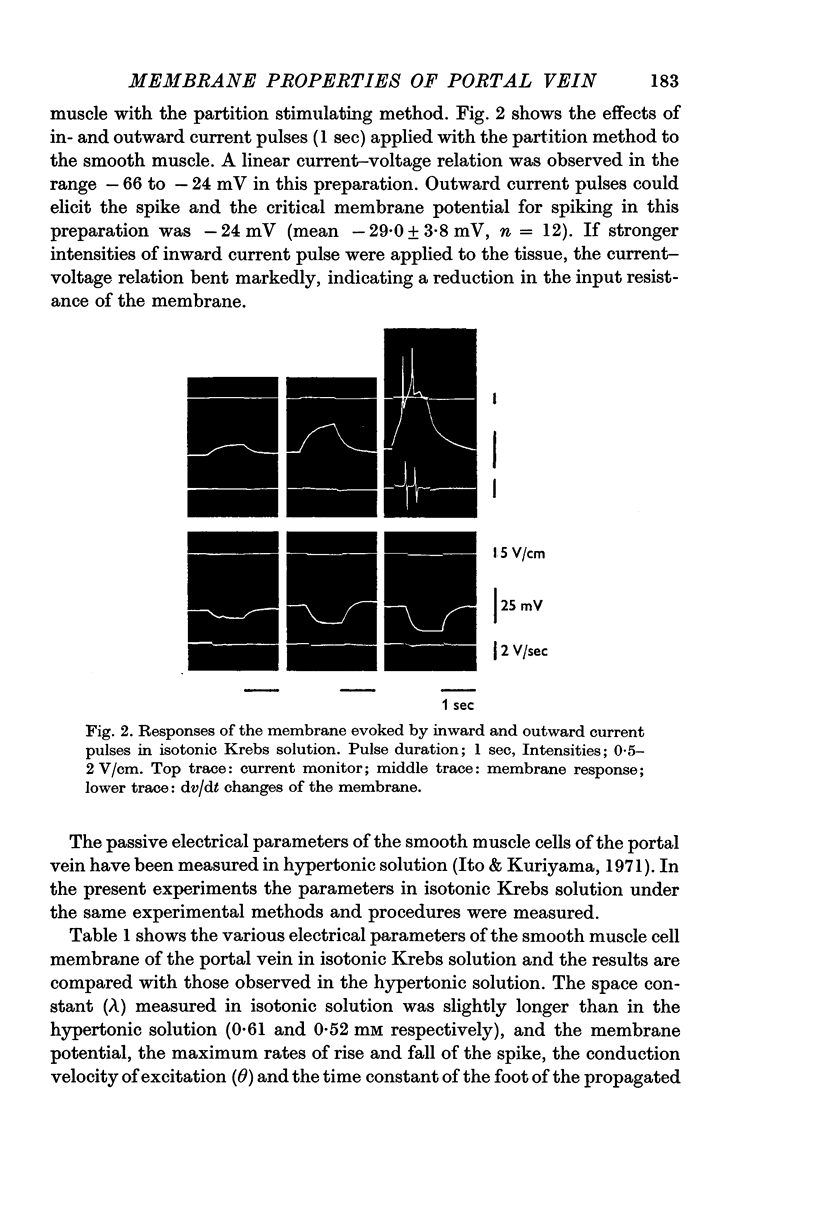
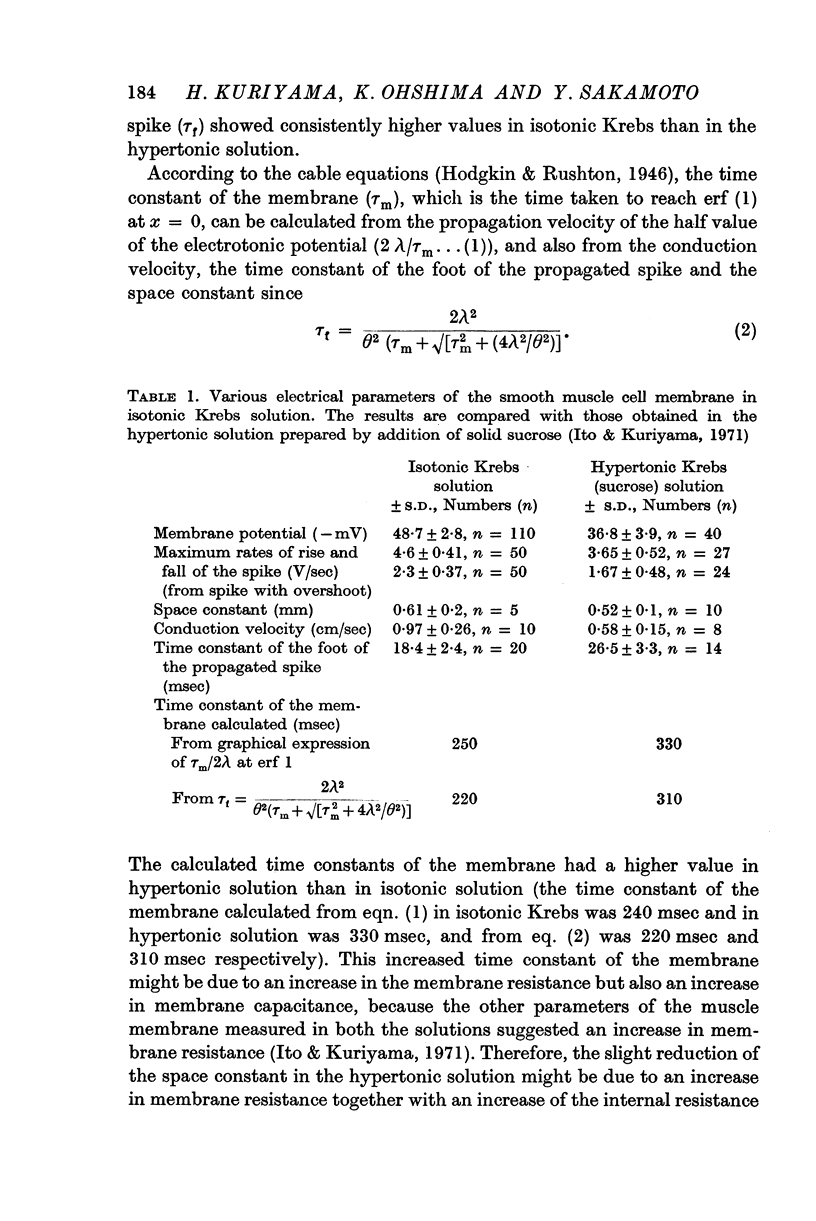
1. In isotonic Krebs solution, the membrane potential (-48·7 mV), the maximum rates of rise and fall of the spike (4·6 and 2·3 V/sec respectively), the space constant (0·61 mm), the conduction velocity of excitation (0·97 cm/sec) and the time constant of the foot of the propagated spike (18·4 msec) were measured.
2. The various parameters of the muscle membrane in the isotonic solution were compared with those in the hypertonic solution prepared by the addition of solid sucrose (twice the normal tonicity).
3. When the muscles were perfused with hypertonic solution, marked depolarization of the membrane and increased membrane resistance occurred. These were probably due to reduction of the K permeability, increased internal resistance of the muscle and shrinkage of the muscle fibre.
4. The membrane potential in isotonic and hypertonic solutions was analysed into two components, i.e. the metabolic (electrogenic Na-pump) and the ionic (electrical diffusion potential) component in the various environmental conditions.
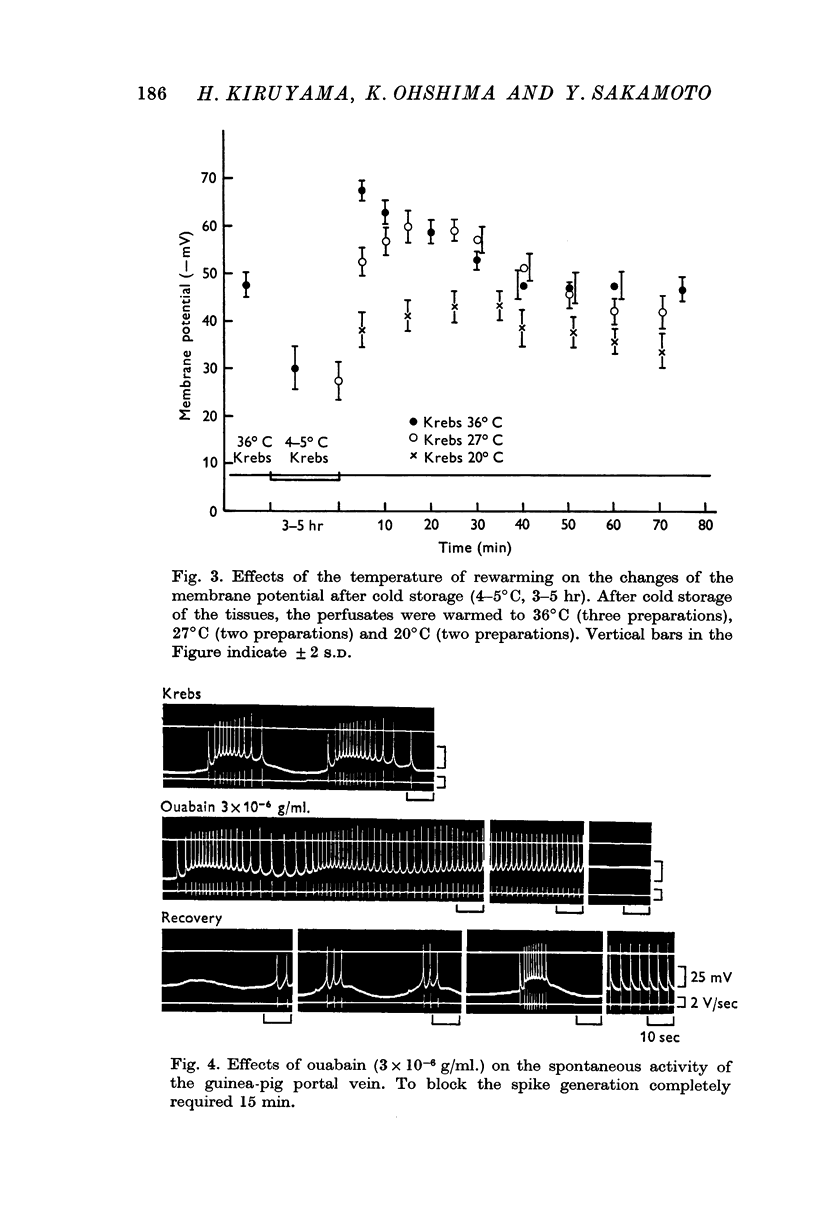
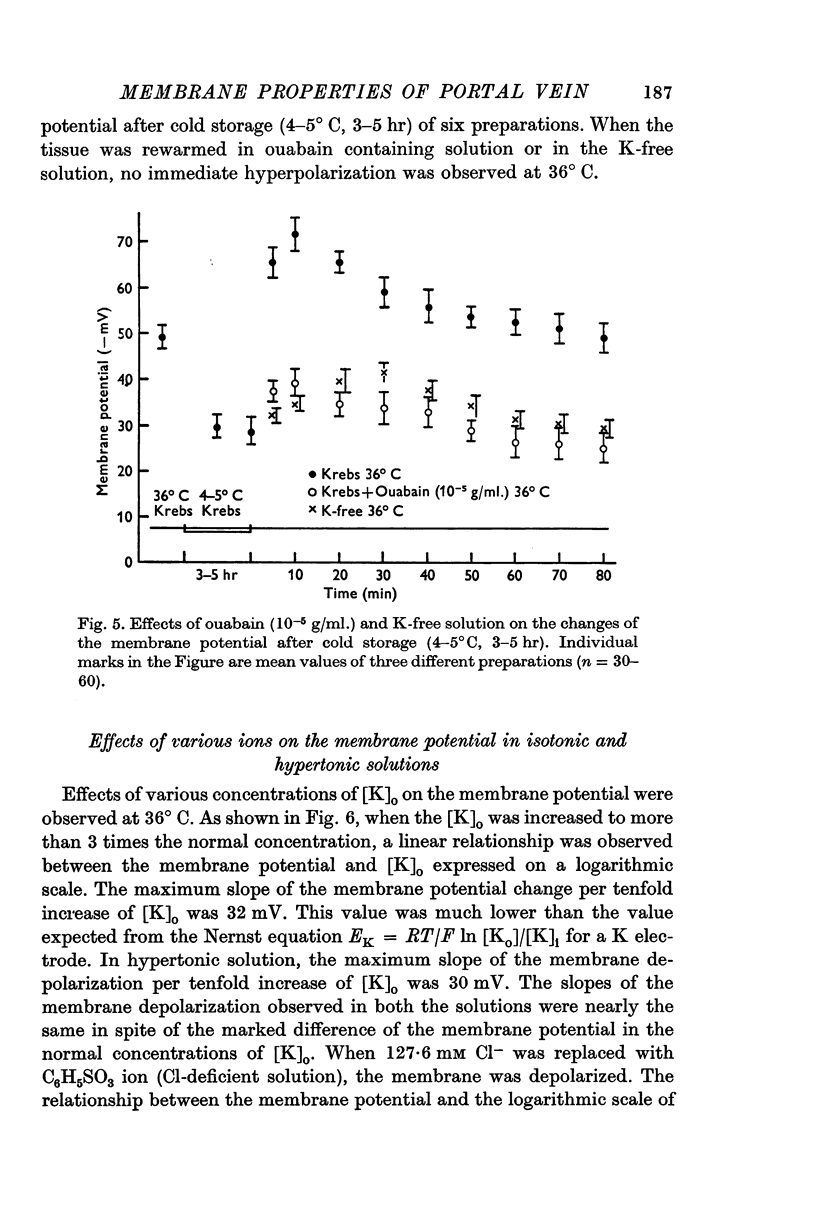
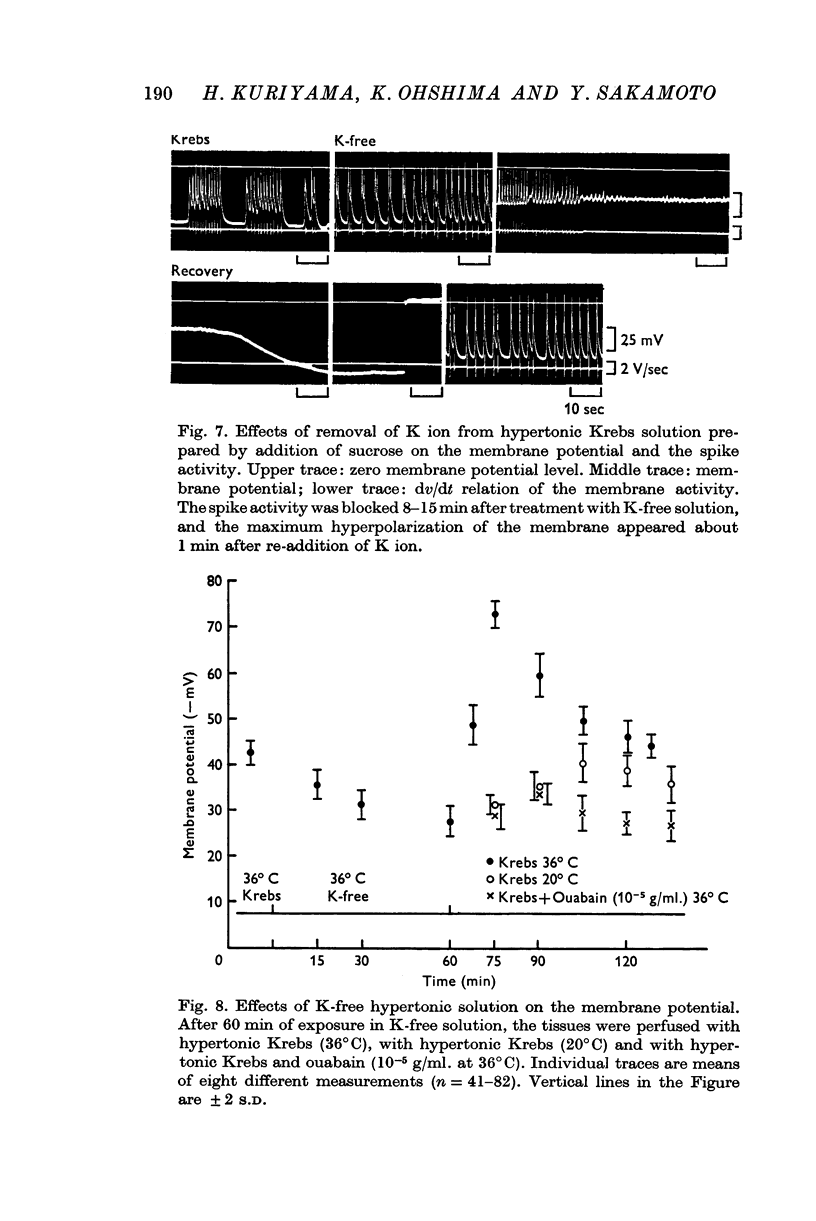
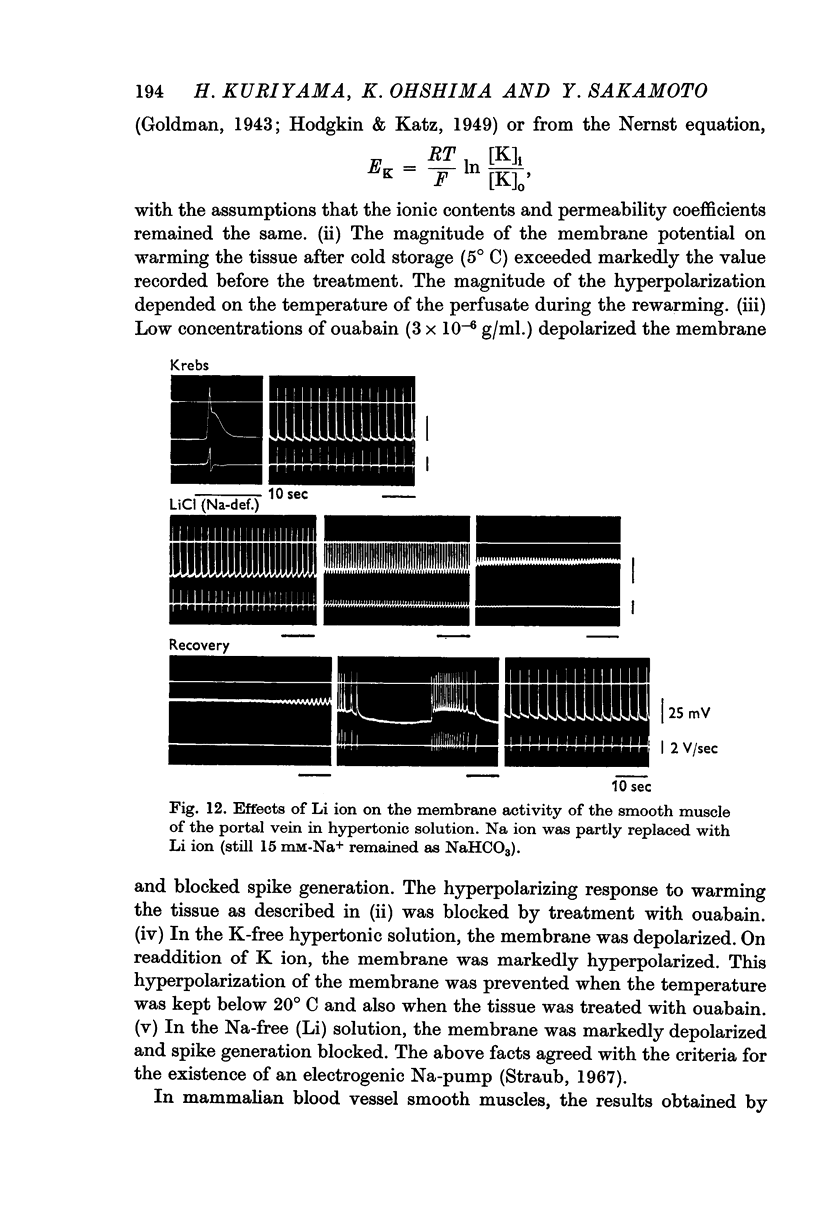
(a) In isotonic and hypertonic solutions, the membrane was depolarized by lowering the temperature or by removal of K ion from the solutions. When the tissues were rewarmed or on readdition of K ion, the membrane was markedly hyperpolarized. These hyperpolarizations of the membrane were suppressed by treatment with ouabain (10-5 g/ml.), by warming to only 20° C and by K-free solution.
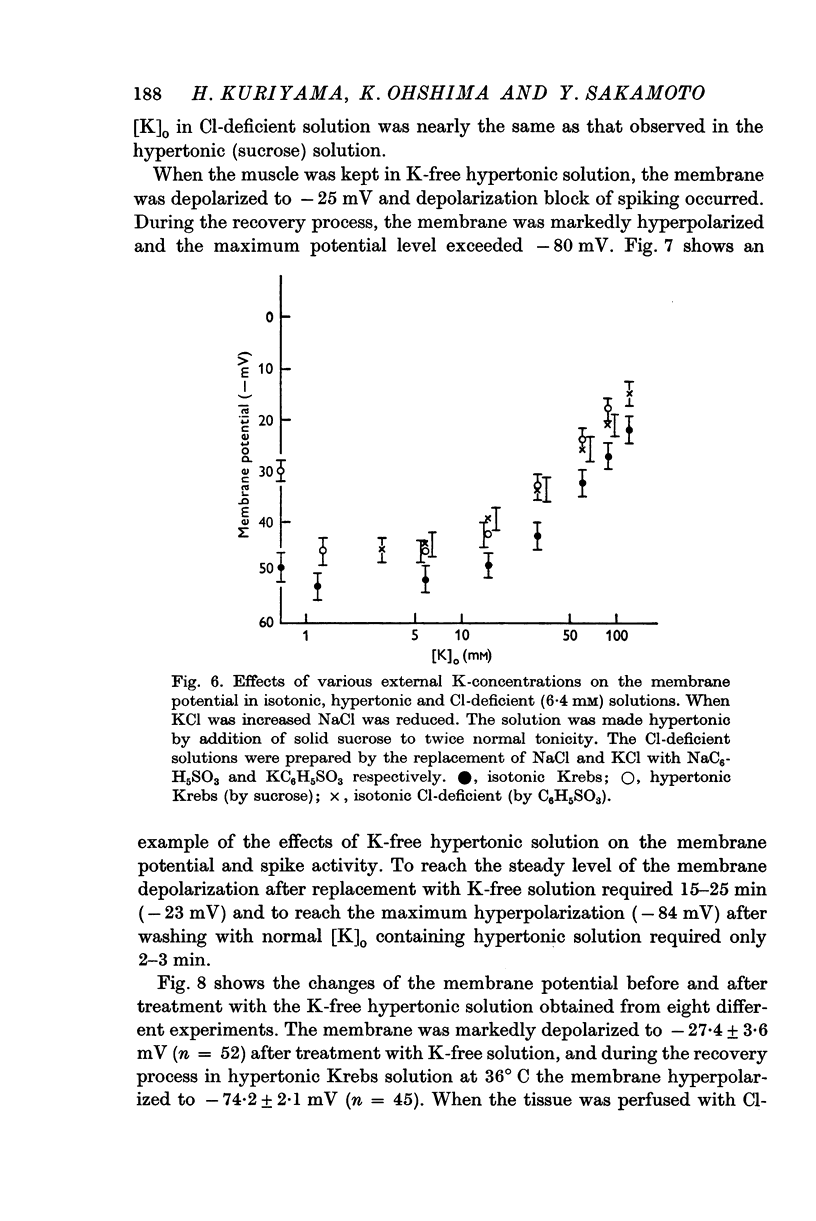
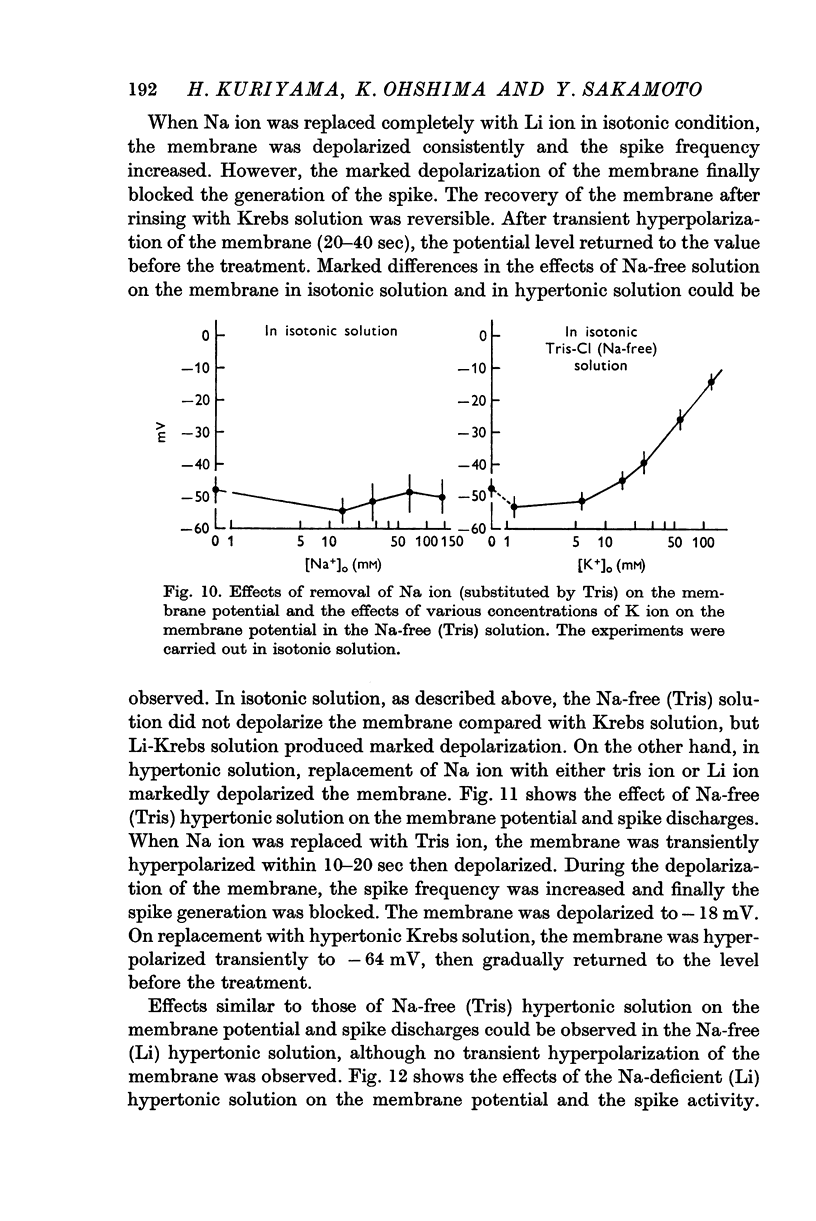
(b) The relationships between the membrane potential and the [K]o in isotonic Krebs, in the hypertonic (sucrose) Krebs, in the Na-free (Tris) Krebs and in the Cl-deficient (C6H5SO3) Krebs were observed. The maximum slopes of the membrane depolarization against tenfold changes of [K]o were much lower than that expected if it behaved like a K electrode.
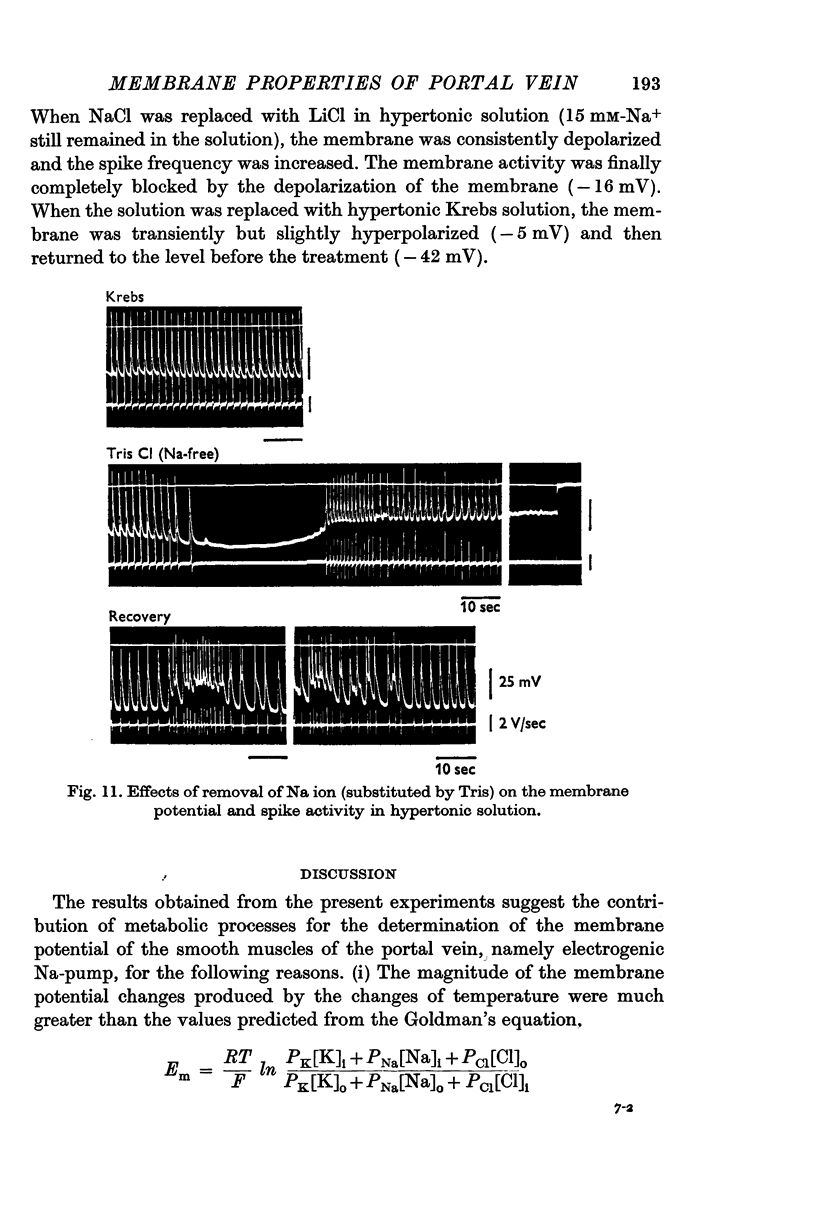
(c) In Na-free (Tris) solution, the membrane was not depolarized in isotonic condition but it was depolarized in hypertonic condition.
5. The low membrane potential in hypertonic solution (-37 mV) compared with isotonic solution (-49 mV) was thought to be mainly due to suppression of K permeability of the membrane and not due to suppression of the metabolic component.
The electrogenic Na-pump and the membrane potential of the portal vein was discussed in relation to other excitable cell membranes.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvill A., Johansson B., Jonsson O. Effects of hyperosmolarity on the volume of vascular smooth muscle cells and the relation between cell volume and muscle activity. Acta Physiol Scand. 1969 Mar;75(3):484–495. doi: 10.1111/j.1748-1716.1969.tb04402.x. [DOI] [PubMed] [Google Scholar]
- Axelsson J., Wahlström B., Johansson B., Jonsson O. Influence of the ionic environment on spontaneous electrical and mechanical activity of the rat portal vein. Circ Res. 1967 Nov;21(5):609–618. doi: 10.1161/01.res.21.5.609. [DOI] [PubMed] [Google Scholar]
- BARR L., HEADINGS V. E., BOHR D. F. Potassium and the recovery of arterial smooth muscle after cold storage. J Gen Physiol. 1962 Sep;46:19–33. doi: 10.1085/jgp.46.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kuriyama H. Membrane potential and ion content in the smooth muscle of the guinea-pig's taenia coli at different external potassium concentrations. J Physiol. 1966 May;184(1):120–130. doi: 10.1113/jphysiol.1966.sp007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E. Effect of sympathomimetic amines and angiotensin on active ion transport in smooth muscles. Arch Int Pharmacodyn Ther. 1965 Nov;158(1):131–138. [PubMed] [Google Scholar]
- Garrahan P., Villamil M. F., Zadunaisky J. A. Sodium exchange and distribution in the arterial wall. Am J Physiol. 1965 Nov;209(5):955–960. doi: 10.1152/ajplegacy.1965.209.5.955. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen K., von Loh D. Intracelluläre Potentialmessungen zur normalen Spontanaktivität der isolierten Portalvene des Meerschweinches. Pflugers Arch. 1970;319(1):82–100. doi: 10.1007/BF00586430. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haljamäe H., Johansson B., Jonsson O., Röckert H. The distribution of sodium, potassium and chloride in the smooth muscle of the rat portal vein. Acta Physiol Scand. 1970 Feb;78(2):255–268. doi: 10.1111/j.1748-1716.1970.tb04661.x. [DOI] [PubMed] [Google Scholar]
- Holman M. E. Electrophysiology of vascular smooth muscle. Ergeb Physiol. 1969;61:137–177. doi: 10.1007/BFb0111448. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H. Membrane properties of the smooth-muscle fibres of the guinea-pig portal vein. J Physiol. 1971 May;214(3):427–441. doi: 10.1113/jphysiol.1971.sp009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Mekata F. Biophysical properties of the longitudinal smooth muscle of the guinea-pig rectum. J Physiol. 1971 Feb;212(3):667–683. doi: 10.1113/jphysiol.1971.sp009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. The binding of tritiated ouabain to mammalian non-myelinated nerve fibres. J Physiol. 1970 Apr;207(2):529–537. doi: 10.1113/jphysiol.1970.sp009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton R. B. An investigation of the electrogenic sodium pump in snail neurones, using the constant-field theory. J Exp Biol. 1969 Aug;51(1):181–201. doi: 10.1242/jeb.51.1.181. [DOI] [PubMed] [Google Scholar]
- Osa T., Kuriyama H. The membrane properties and decremental conduction of excitation in the fundus of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):626–639. doi: 10.2170/jjphysiol.20.626. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The dependence on external cations of the oxygen consumption of mammalian non-myelinated fibres at rest and during activity. J Physiol. 1968 May;196(1):163–181. doi: 10.1113/jphysiol.1968.sp008501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S. I. The sodium-potassium exchange pump: relation of metabolism to electrical properties of the cell. I. Theory. Biophys J. 1970 Mar;10(3):246–259. doi: 10.1016/S0006-3495(70)86297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Kuriyama H. The relationship between the electrical and mechanical activity of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):640–656. doi: 10.2170/jjphysiol.20.640. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev. 1968 Dec;20(4):197–272. [PubMed] [Google Scholar]
- Steedman W. M. Micro-electrode studies on mammalian vascular muscle. J Physiol. 1966 Oct;186(2):382–400. doi: 10.1113/jphysiol.1966.sp008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R. W. Der Einfluss von Ionenpumpen auf das Membranpotential. Bull Schweiz Akad Med Wiss. 1967 Dec;23(3):271–278. [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Spike propagation in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Aug;191(3):517–527. doi: 10.1113/jphysiol.1967.sp008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIDLAKOVA M., KLEINZELLER A. Active transport of electrolytes in the smooth muscle of pigeon gizzard and rabbit aorta. Physiol Bohemoslov. 1963;12:202–207. [PubMed] [Google Scholar]


