Abstract
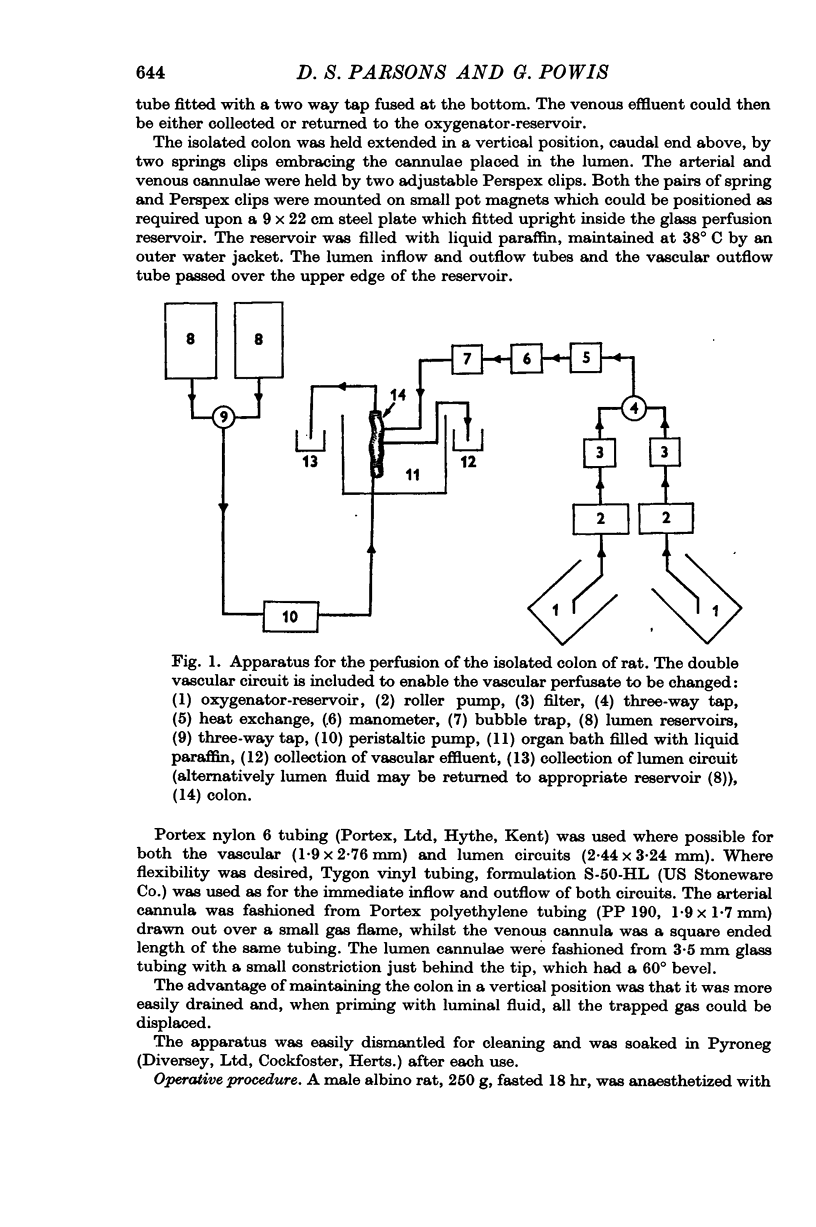
1. A technique is described for the perfusion of the isolated colon of the rat, involving the infusion of an appropriate fluid through both the inferior and the superior mesenteric arteries. During neither the preparation nor the subsequent perfusion is the colon without an adequate supply of oxygen. The preparation remains histologically intact and metabolically viable and is capable of actively transporting ions for up to 5 hr.
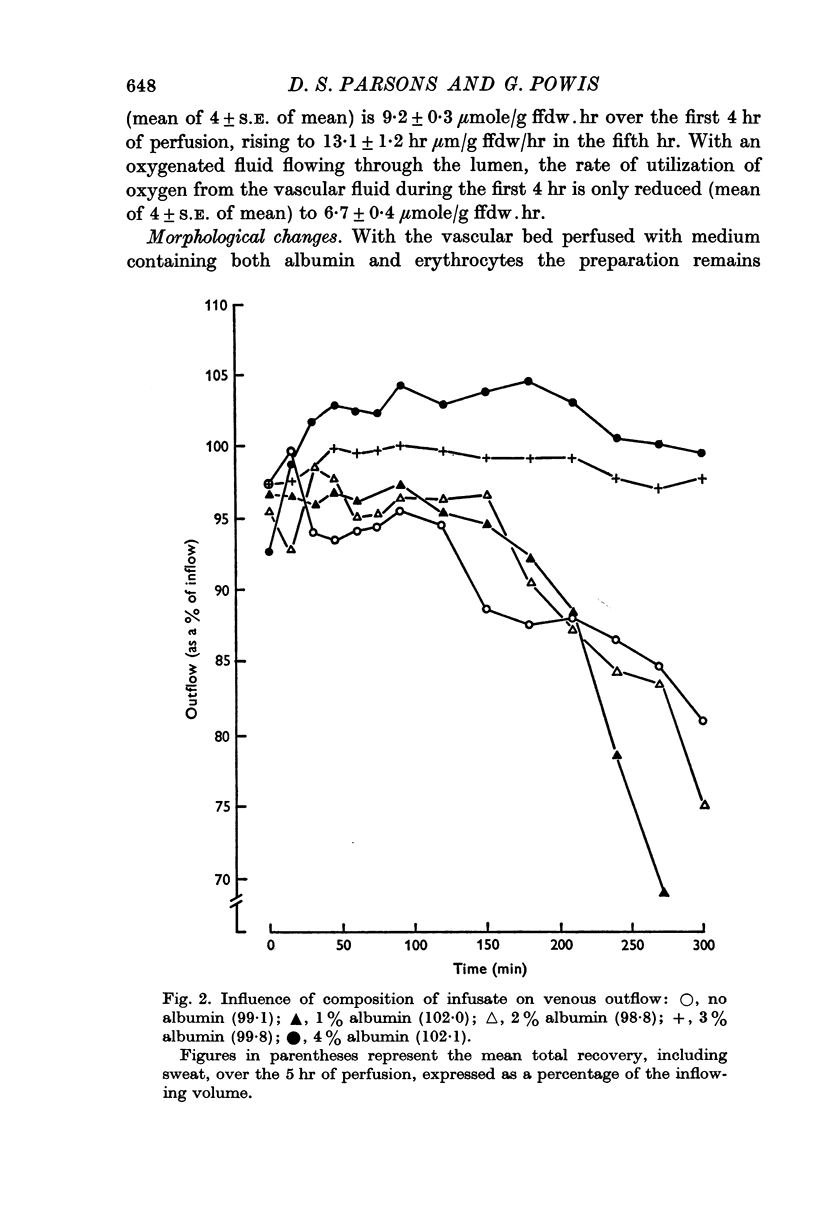
2. The addition of at least 3 g albumin/100 ml. perfusate is necessary to prevent the formation of large quantities of serosal exudate. With erythrocytes added to the vascular perfusate the preparation appears to be adequately oxygenated as judged by measurements of the rate of glycolysis. The mean rate of oxygen utilization over 4 hr is 9·2 ± 0·3 (4) μmole. hr-1.g-1 fat free dry weight.
3. Ion transport rates approaching those found in vivo are found only after the administration of an antihistamine substance to the colon donor rat before operation. In the absence of an antihistamine substance there appears to be an ultrafiltration of the plasma fluid into the lumen.
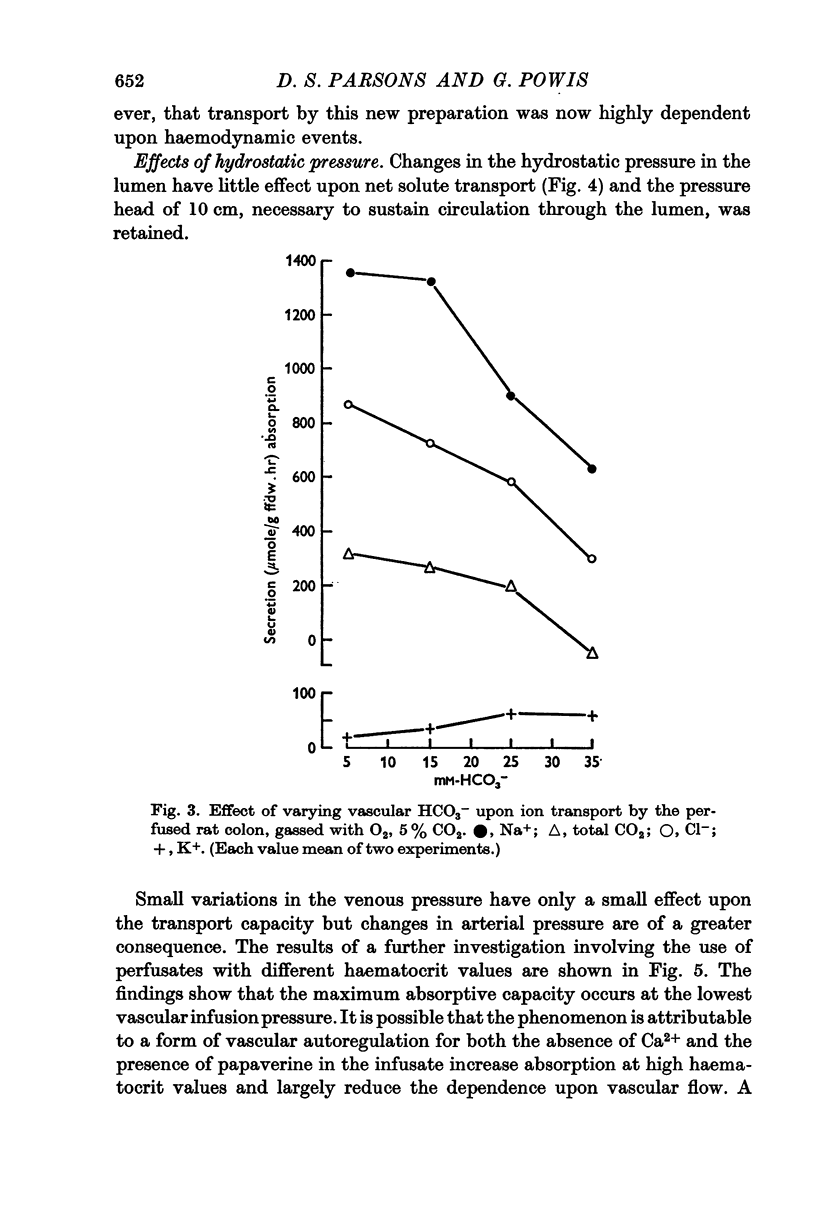
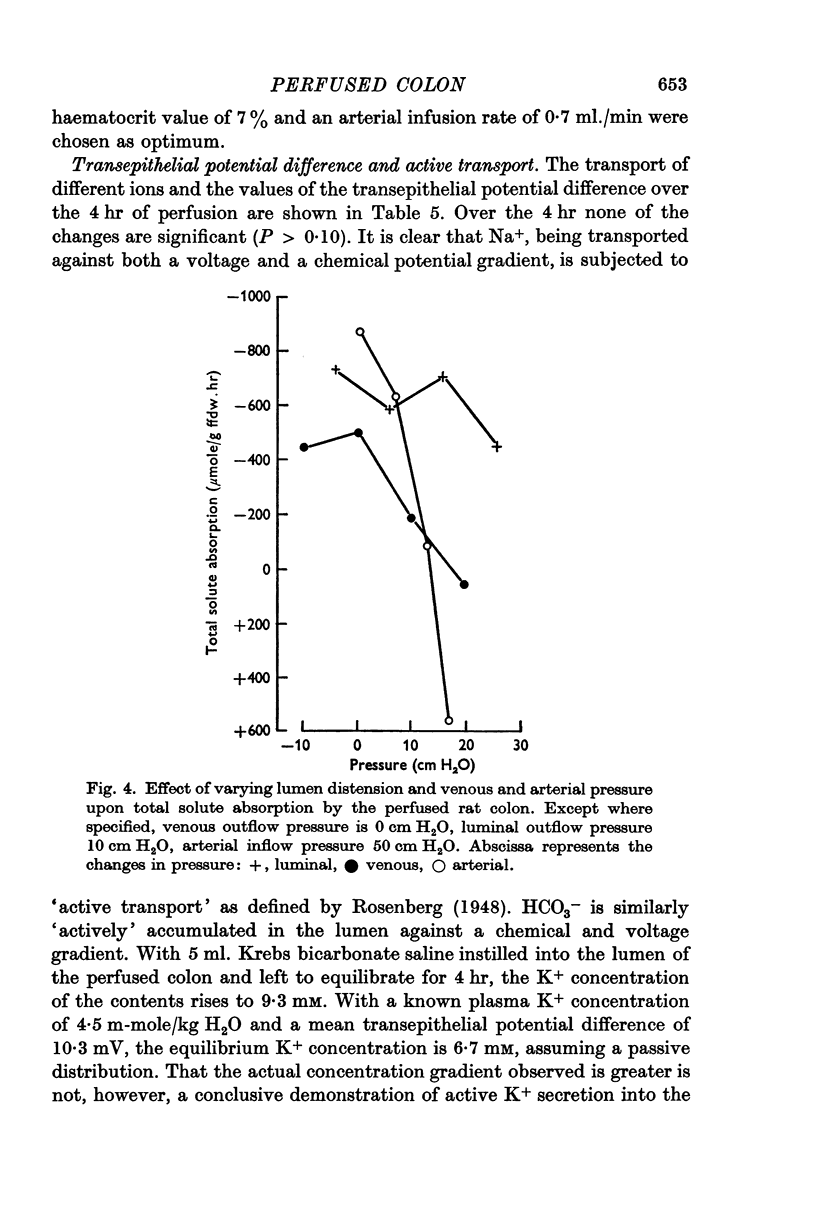
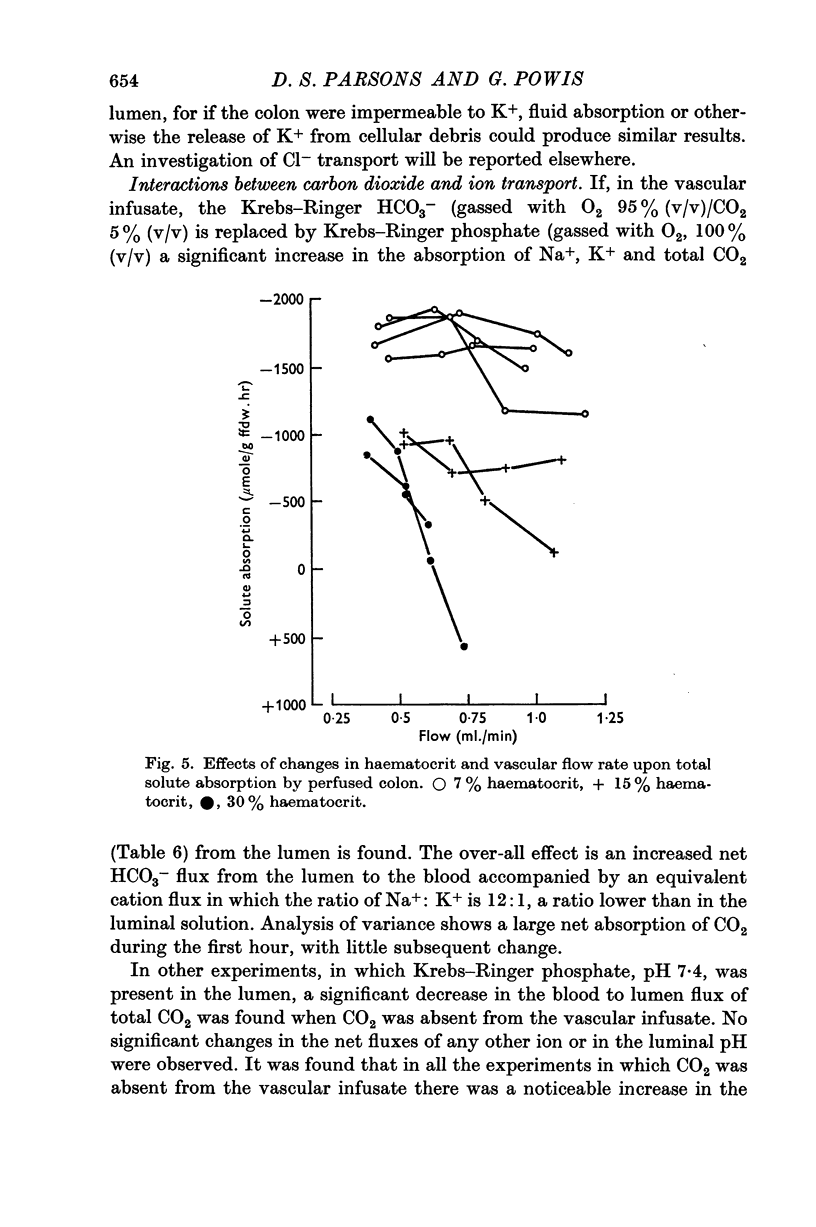
4. Vasodilatory substances accumulate in the recycled perfusate. In a `single pass' perfusion, the transport capacity of the preparation decreases at high perfusion pressures. It is suggested that this is due to some form of autoregulation whereby perfusate is shunted away from the epithelium into deeper layers as the pressure is increased.
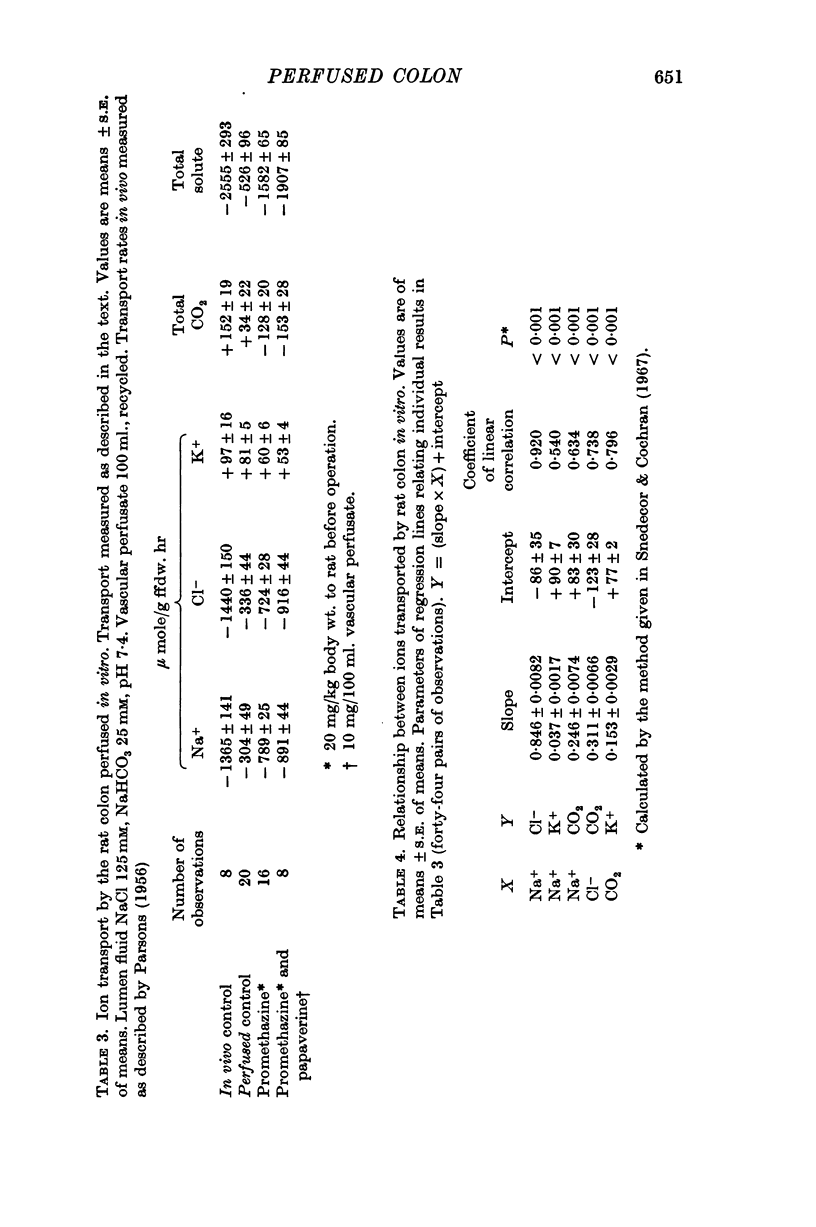
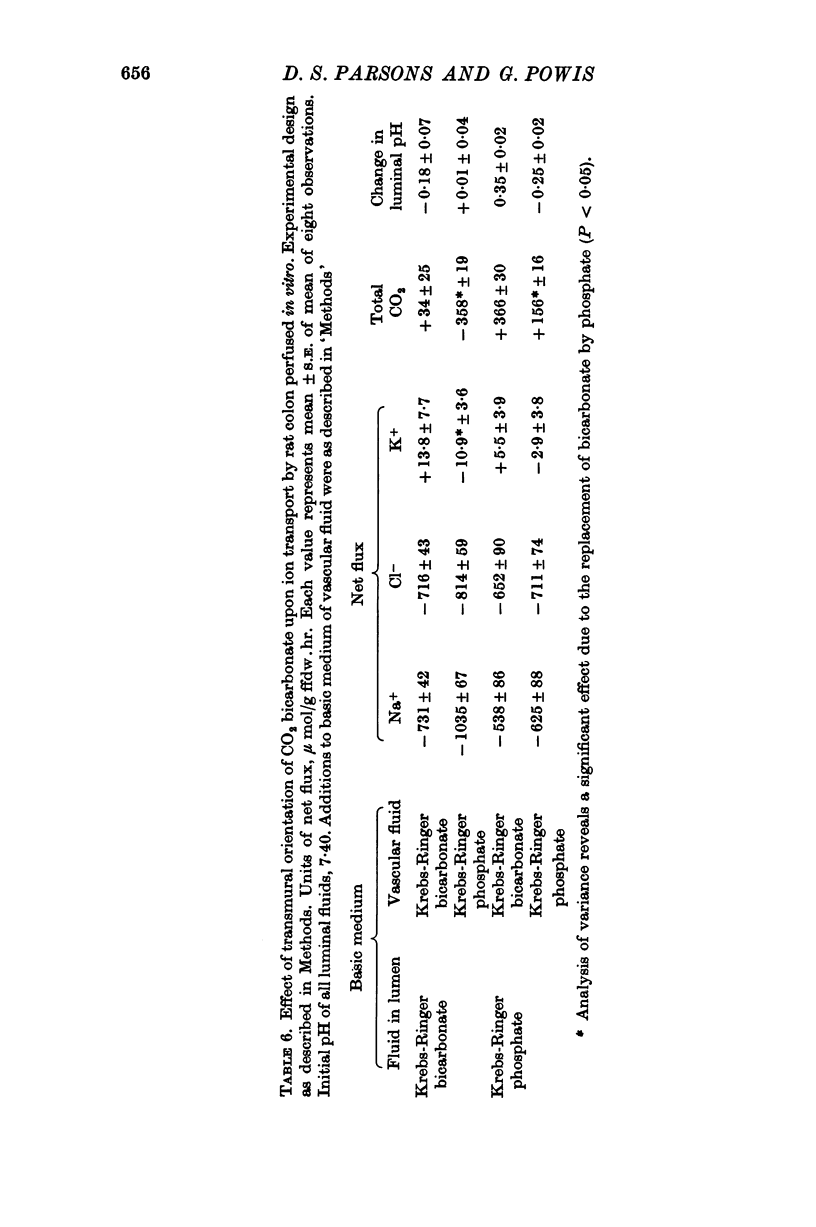
5. With CO2 absent from the vascular infusate there is an increase in the net lumen to blood flux of total CO2. This increased flux is accompanied by an equivalent amount of cation, comprising Na+ and K+ in the ratio of 12:1.
6. The presence of ammonium in the lumen, a physiological constituent of the contents of rat distal colon in vivo, has a marked inhibitory effect upon the secretion of CO2 into the contents of the lumen of the colon.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anrep G. V., von Saalfeld E. The blood flow through the skeletal muscle in relation to its contraction. J Physiol. 1935 Nov 22;85(3):375–399. doi: 10.1113/jphysiol.1935.sp003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNSTEIN R. E. Potassium and sodium balance in mammalian red cells. Science. 1954 Sep 17;120(3116):459–460. doi: 10.1126/science.120.3116.459. [DOI] [PubMed] [Google Scholar]
- Boullin D. J., Costa E., Brodie B. B. Evidence that blockade of adrenergic receptors causes overflow of norepinephrine in cats colon after nerve stimulation. J Pharmacol Exp Ther. 1967 Jul;157(1):125–134. [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The polarographic determination of the respiration of the small intestine of the rat. Biochim Biophys Acta. 1965 Oct 18;107(3):397–404. doi: 10.1016/0304-4165(65)90183-2. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN I. L., HOGBEN C. A. Ionic transfer across the isolated frog large intestine. J Gen Physiol. 1959 Jan 20;42(3):461–473. doi: 10.1085/jgp.42.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., SCHWARTZ G. F. Na, Cl, and water transport by rat colon. J Gen Physiol. 1960 Jan;43:555–571. doi: 10.1085/jgp.43.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'AGOSTINO A., LEADBETTER W. F., SCHWARTZ W. B. Alterations in the ionic composition of isotonic saline solution instilled into the colon. J Clin Invest. 1953 May;32(5):444–448. doi: 10.1172/JCI102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F., Weil-Malherbe H. Metabolism of normal and tumour tissue: The metabolism of intestinal mucous membrane. Biochem J. 1941 Jan;35(1-2):7–15. doi: 10.1042/bj0350007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. The gradient of electrical potential difference and of sodium and potassium of the gut contents along the caecum and colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):571–588. doi: 10.1113/jphysiol.1967.sp008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of sodium and secretion of potassium and bicarbonate by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):589–602. doi: 10.1113/jphysiol.1967.sp008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. A preparation of surviving rat small intestine for the study of absorption. J Physiol. 1949 Dec 15;110(1-2):36-46, pl. doi: 10.1113/jphysiol.1949.sp004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J., Ussing H. H., Wieth J. O. The effects of CO2 and hydrogen ions on active Na transport in the isolated frog skin. Acta Physiol Scand. 1967 Sep;71(1):65–76. doi: 10.1111/j.1748-1716.1967.tb03710.x. [DOI] [PubMed] [Google Scholar]
- GARRY R. C., HOLMES M., WISHART M. An in vitro preparation of the rabbit colon perfused through the inferior mesenteric artery. J Physiol. 1957 Dec 3;139(2):3–4P. [PubMed] [Google Scholar]
- Gonzalez C. F., Shamoo Y. E., Brodsky W. A. The accelerating effect of serosal HCO3- on Na+ transport in short-circuited turtle bladders. Biochim Biophys Acta. 1969;193(2):403–418. doi: 10.1016/0005-2736(69)90200-4. [DOI] [PubMed] [Google Scholar]
- Grenier J. F., Wong P., Kachelhoffer J., Barth A. M., Baruthio F., Clavert A., Weiss A. G. Les perfusions d'intestin isolé chez le chien. Mise au point d'une technique. Son intérêt. C R Seances Soc Biol Fil. 1967;161(12):2624–2627. [PubMed] [Google Scholar]
- Haddy F. J., Scott J. B. Metabolically linked vasoactive chemicals in local regulation of blood flow. Physiol Rev. 1968 Oct;48(4):688–707. doi: 10.1152/physrev.1968.48.4.688. [DOI] [PubMed] [Google Scholar]
- JELLIFFE R. W., WOLF C. R., BERNE R. M., ECKSTEIN R. W. Absence of vasoactive and cardiotropic substances in coronary sinus blood of dogs. Circ Res. 1957 Jul;5(4):382–387. doi: 10.1161/01.res.5.4.382. [DOI] [PubMed] [Google Scholar]
- JUDAH J. D. Action of antihistamine and other drugs on ion and water movements in cells and mitochondria. Fed Proc. 1962 Nov-Dec;21:1097–1099. [PubMed] [Google Scholar]
- KINNEY V. R., CODE C. F. CANINE ILEAL CHLORIDE ABSORPTION: EFFECT OF CARBONIC ANHYDRASE INHIBITOR ON TRANSPORT. Am J Physiol. 1964 Nov;207:998–1004. doi: 10.1152/ajplegacy.1964.207.5.998. [DOI] [PubMed] [Google Scholar]
- Kavin H., Levin N. W., Stanley M. M. Isolated perfused rat small bowel--technic, studies of viability, glucose absorption. J Appl Physiol. 1967 Mar;22(3):604–611. doi: 10.1152/jappl.1967.22.3.604. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Duncan K. M. Lymphatic and venous transport of water from rat jejunum: a vascular perfusion study. Gastroenterology. 1968 Apr;54(4):559–567. [PubMed] [Google Scholar]
- MOHAMED M. S., BEAN J. W. Local and general alterations of blood CO2 and influence of intestinal motility in regulation of intestinal blood flow. Am J Physiol. 1951 Nov;167(2):413–425. doi: 10.1152/ajplegacy.1951.167.2.413. [DOI] [PubMed] [Google Scholar]
- Mossberg S. M., Ross G. Ammonia movement in the small intestine: preferential transport by the ileum. J Clin Invest. 1967 Apr;46(4):490–498. doi: 10.1172/JCI105551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS D. S., PATERSON C. R. FLUID AND SOLUTE TRANSPORT ACROSS FAT COLONIC MUCOSA. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:220–231. doi: 10.1113/expphysiol.1965.sp001784. [DOI] [PubMed] [Google Scholar]
- PARSONS D. S., VAN ROSSUM G. D. Post natal changes in the water and electrolyte content of rat liver. Q J Exp Physiol Cogn Med Sci. 1961 Oct;46:353–368. doi: 10.1113/expphysiol.1961.sp001554. [DOI] [PubMed] [Google Scholar]
- Parsons D. S., Prichard J. S. A preparation of perfused small intestine for the study of absorption in amphibia. J Physiol. 1968 Sep;198(2):405–434. doi: 10.1113/jphysiol.1968.sp008614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. S. Salt and water absorption by the intestinal tract. Br Med Bull. 1967 Sep;23(3):252–257. doi: 10.1093/oxfordjournals.bmb.a070566. [DOI] [PubMed] [Google Scholar]
- Phemister D. B., Handy J. Vascular properties of traumatised and laked bloods. J Physiol. 1927 Nov 21;64(2):155–173. doi: 10.1113/jphysiol.1927.sp002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt H. S. The metabolism of the small intestine. Oxygen uptake and L-lactate production along the length of the small intestine of the rat and guinea pig. Comp Biochem Physiol. 1968 Mar;24(3):745–761. doi: 10.1016/0010-406x(68)90787-1. [DOI] [PubMed] [Google Scholar]
- Sladen G. E., Dawson A. M. Effect of bicarbonate on sodium absorption by the human jejunum. Nature. 1968 Apr 20;218(5138):267–268. doi: 10.1038/218267a0. [DOI] [PubMed] [Google Scholar]
- Sladen G. E., Parsons D. S., Dupre J. Effects of bicarbonate on intestinal absorption. Gut. 1968 Dec;9(6):731–731. [PubMed] [Google Scholar]
- Swallow J. H., Code C. F. Intestinal transmucosal fluxes of bicarbonate. Am J Physiol. 1967 Mar;212(3):717–723. doi: 10.1152/ajplegacy.1967.212.3.717. [DOI] [PubMed] [Google Scholar]
- WILSON T. H. The role of lactic acid production in glucose absorption from the intestine. J Biol Chem. 1956 Oct;222(2):751–763. [PubMed] [Google Scholar]
- WRONG O., METCALFE-GIBSON A., MORRISON R. B., NG S. T., HOWARD A. V. IN VIVO DIALYSIS OF FAECES AS A METHOD OF STOOL ANALYSIS. I. TECHNIQUE AND RESULTS IN NORMAL SUBJECTS. Clin Sci. 1965 Apr;28:357–375. [PubMed] [Google Scholar]
- Whittam R., Wheeler K. P. Transport across cell membranes. Annu Rev Physiol. 1970;32:21–60. doi: 10.1146/annurev.ph.32.030170.000321. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E., Ganote C. E. Vascular perfusion of isolated rat gut: norepinephrine and glucocorticoid requirement. Am J Physiol. 1970 Jan;218(1):197–204. doi: 10.1152/ajplegacy.1970.218.1.197. [DOI] [PubMed] [Google Scholar]


