Abstract
1. The production of heat and (internal) work and the changes in the amount of phosphocreatine (PCr), ATP, inorganic phosphate (Pi) and sometimes lactate have been measured from moment to moment during and after tetanic isometric contractions of isolated frog muscles at 0° C.
2. Heat production was measured by thermopiles and a novel apparatus was employed for freezing the muscles rapidly at a chosen instant so as to halt the chemical processes before analysis.
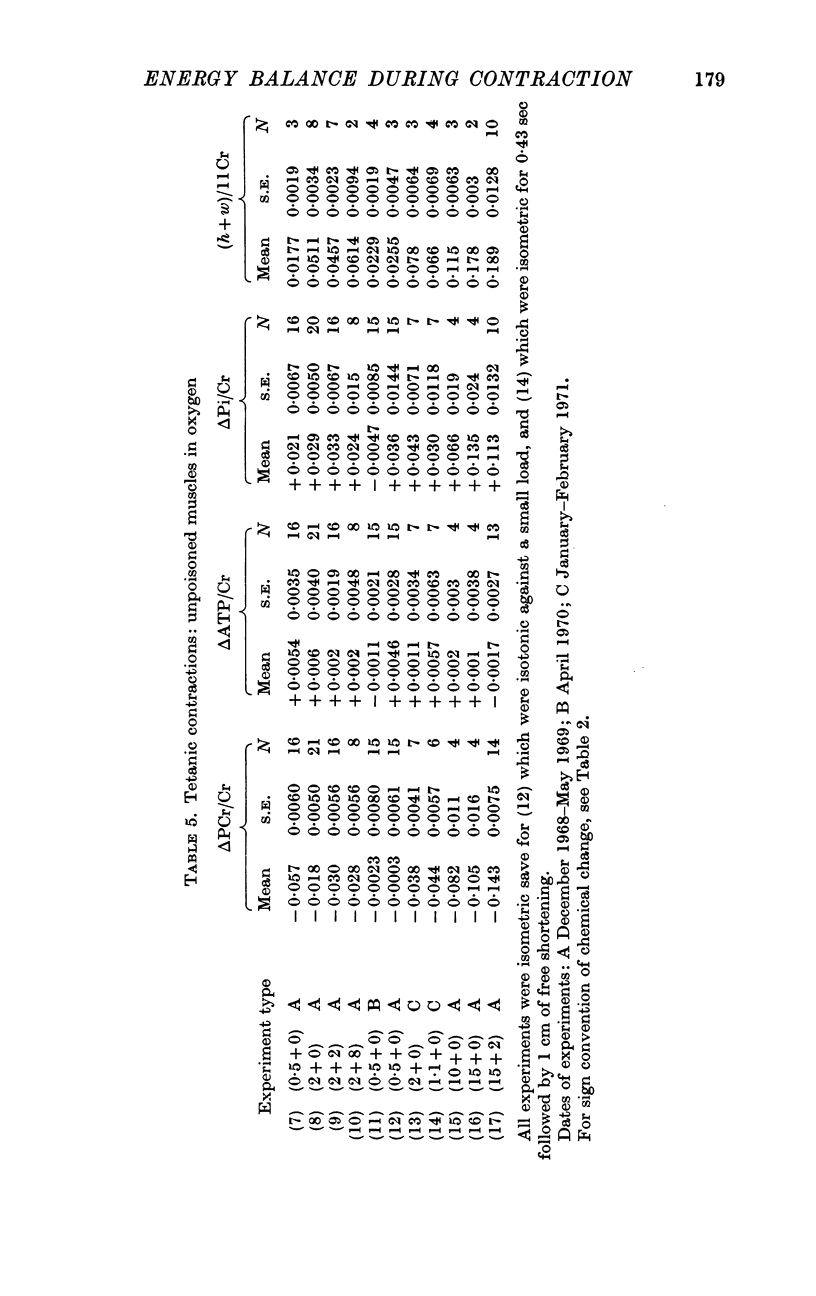
3. Using unpoisoned muscles in oxygen, it was shown that neither oxidative recovery processes nor glycolytic ones led to appreciable restitution of PCr or ATP during 15 sec of contraction. However, clear signs of recovery processes could be seen within a minute. In our preparations artificial `ageing' by storage at low temperature did not interfere with the capacity for glycolysis.
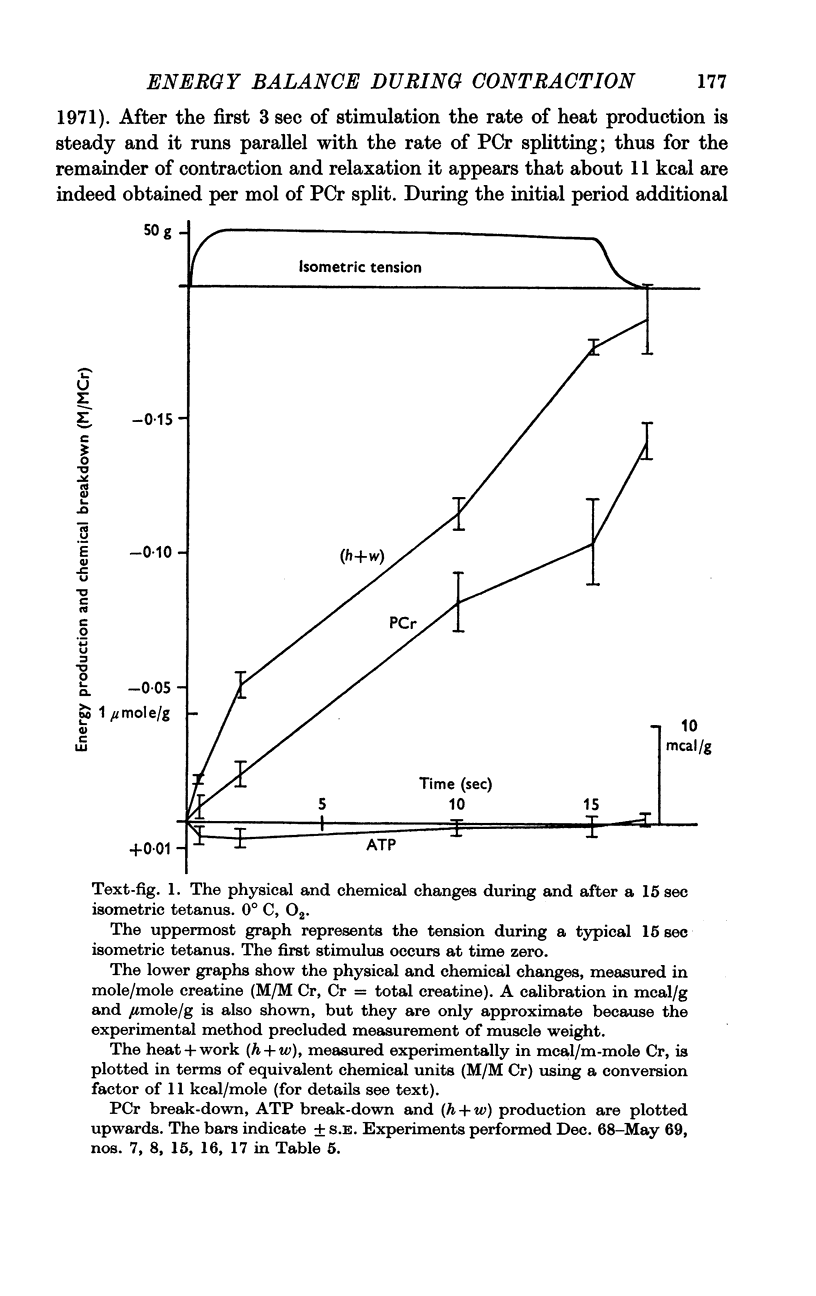
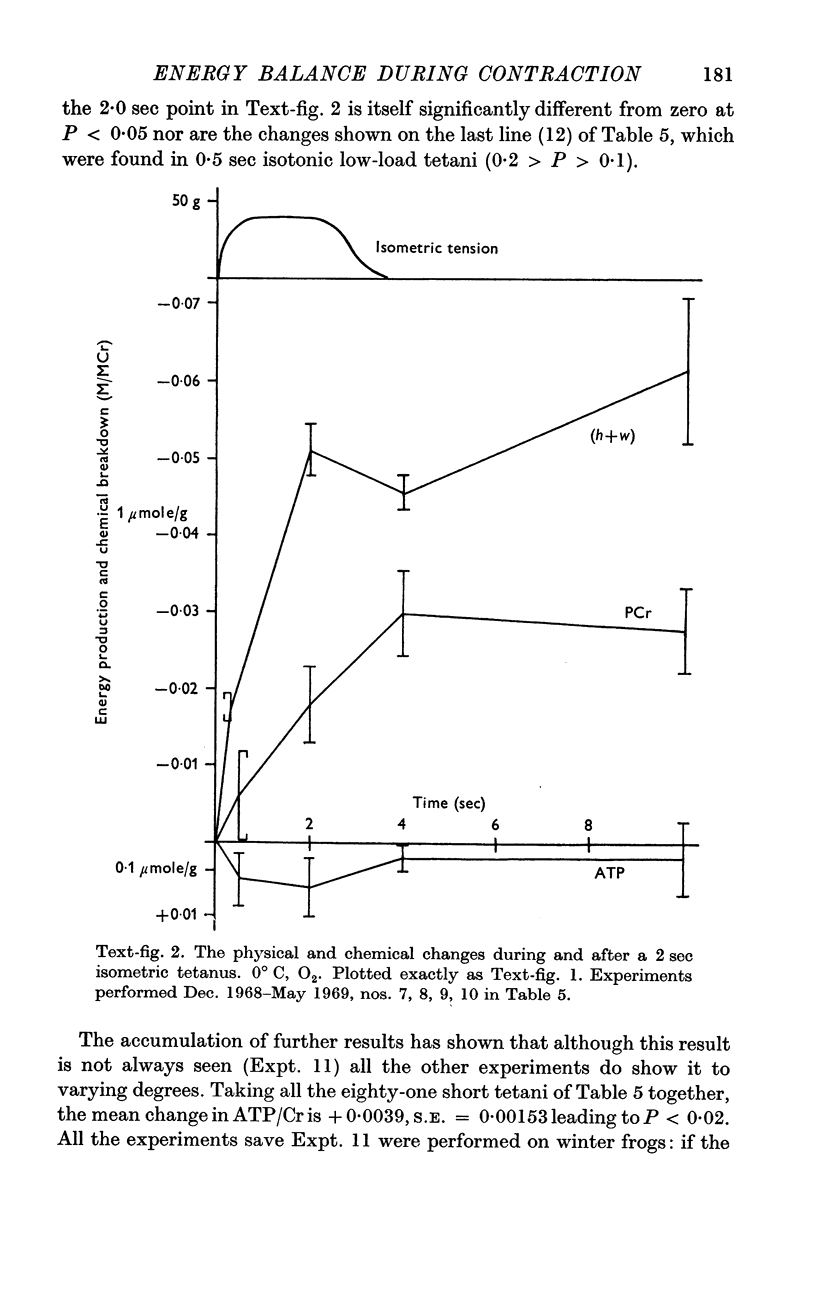
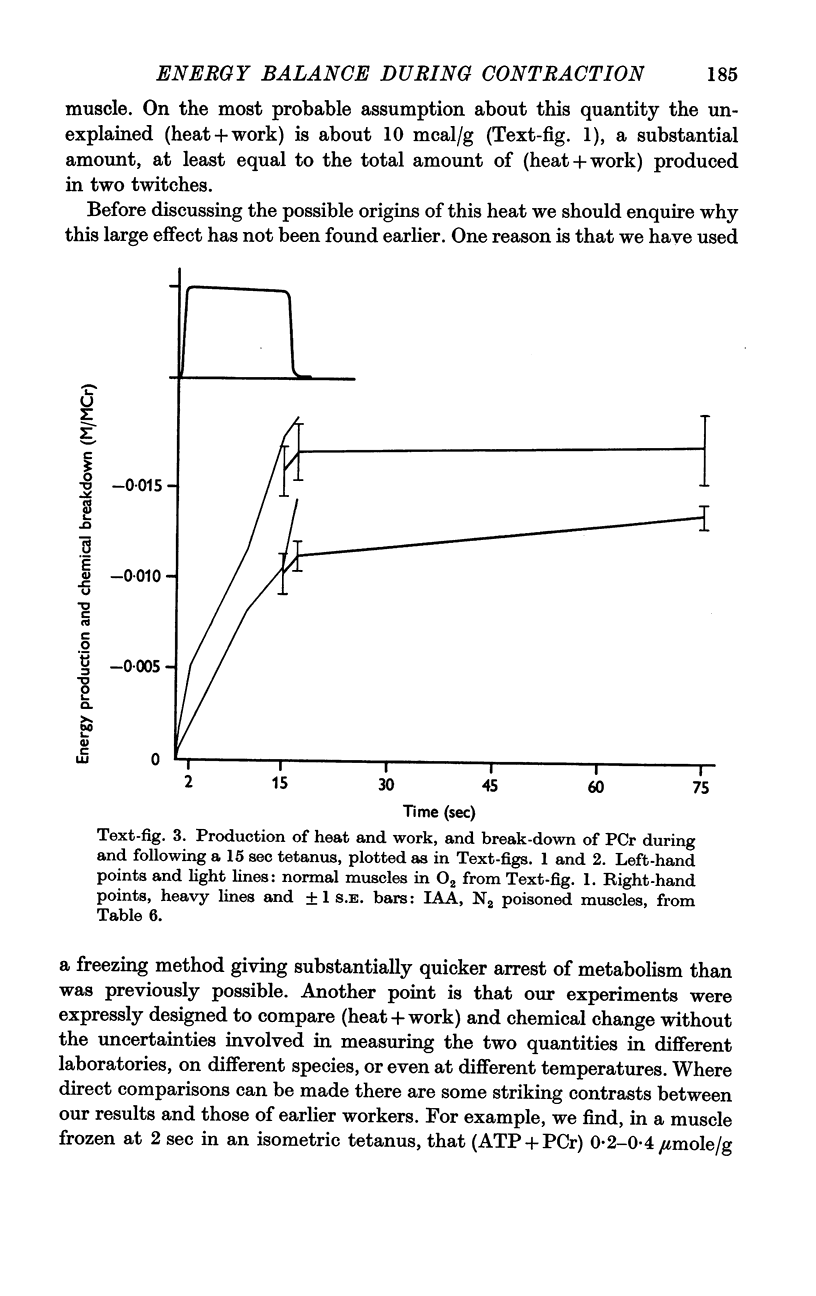
4. Our clearest result was that the break-down of PCr was not nearly large enough to account for the rapid heat production during the first few sec of contraction. By the end of a 15 sec tetanus as much as 10 mcal/g remained unaccounted for.
5. The source of this heat is not clear. At no time is there any sign of net break-down of ATP; indeed there appears to be a slight increase of ATP in the stimulated muscle.
6. Break-down of PCr continues both during relaxation and during the minute following, while the muscle is at rest. Thus during contraction there is heat production without PCr break-down, while subsequently there is PCr break-down without heat production.
Full text
PDF

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bárány M., Bárány K., Bailin G. Reactivity of actomyosin and myosin with 1-fluoro-2,4-dinitrobenzene in vivo and in vitro. Biochim Biophys Acta. 1968 Oct 21;168(2):298–310. doi: 10.1016/0005-2795(68)90152-9. [DOI] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The creatine phosphoryltransfer reaction in iodoacetate-poisoned muscle. J Gen Physiol. 1959 Nov;43:301–313. doi: 10.1085/jgp.43.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The mechanochemistry of muscular contraction. I. The isometric twitch. J Gen Physiol. 1960 Sep;44:33–60. doi: 10.1085/jgp.44.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WEBER A. THE STEADY STATE OF CYTOCHROME B DURING REST AND AFTER CONTRACTION IN FROG SARTORIUS. J Physiol. 1963 Nov;169:263–277. doi: 10.1113/jphysiol.1963.sp007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson F. D., Hardy D., Wilkie D. R. The relation between heat produced and phosphorylcreatine split during isometric contraction of frog's muscle. J Physiol. 1967 Apr;189(2):209–235. doi: 10.1113/jphysiol.1967.sp008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydyńska M., Wilkie D. R. The chemical and energetic properties of muscles poisoned with fluorodinitrobenzene. J Physiol. 1966 Jun;184(3):751–769. doi: 10.1113/jphysiol.1966.sp007946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Le bilan énergétique pendant la contraction musculaire. J Physiol (Paris) 1969;61 (Suppl 2):299–299. [PubMed] [Google Scholar]
- Gilbert C., Kushmerick M. J. Eenergy balance during working contractions of frog muscle. J Physiol. 1970 Sep;210(2):146P–147P. [PubMed] [Google Scholar]
- HILL A. V., WOLEDGE R. C. An examination of absolute values in myothermic measurements. J Physiol. 1962 Jul;162:311–333. doi: 10.1113/jphysiol.1962.sp006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The energy degraded in the recovery processes of stimulated muscles. J Physiol. 1913 Mar 3;46(1):28–80. doi: 10.1113/jphysiol.1913.sp001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. The anaerobic recovery heat production of frog's muscle at 0 degrees C. J Physiol. 1940 Sep 14;98(4):460–466. doi: 10.1113/jphysiol.1940.sp003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOBSIS F. F. Spectrophotometric studies on intact muscle. II. Recovery from contractile activity. J Gen Physiol. 1963 May;46:929–969. doi: 10.1085/jgp.46.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell B. R., Kretzschmar M., Woledge R. C. Length and tension transducers. J Physiol. 1967 Jul;191(1):10P–12P. [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. A new approach to freezing tissues rapidly. J Physiol. 1969 Jun;202(2):66P–67P. [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Larson R. E., Davies R. E. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilization for activation-relaxation processes. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):293–313. doi: 10.1098/rspb.1969.0095. [DOI] [PubMed] [Google Scholar]
- MARECHAL G., MOMMAERTS W. F. The metabolism of phosphocreatine during an isometric tetanus in the frog sartorius muscle. Biochim Biophys Acta. 1963 Feb 19;70:53–67. doi: 10.1016/0006-3002(63)90718-2. [DOI] [PubMed] [Google Scholar]
- MOOS C. CAN CREATINE KINASE PHOSPHORYLATE THE MYOFIBRIL-BOUND NUCLEOTIDE OF MUSCLE:? Biochim Biophys Acta. 1964 Oct 9;93:85–97. doi: 10.1016/0304-4165(64)90263-6. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F., Wallner A. The break-down of adenosine triphosphate in the contraction cycle of the frog sartorius muscle. J Physiol. 1967 Nov;193(2):343–357. doi: 10.1113/jphysiol.1967.sp008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A. Enzymatic fluorometric methods for the microdetermination of hexose phosphates in muscle. J Biol Chem. 1960 Aug;235:2191–2195. [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A. The amount and compartmentalization of adenosine diphosphate in muscle. Biochim Biophys Acta. 1962 Dec 17;65:443–460. doi: 10.1016/0006-3002(62)90447-x. [DOI] [PubMed] [Google Scholar]
- Spronck A. C. Evolution temporelle de l'hydrolyse de la phosphocréatine et de la synthèse d'hexosediphosphate pendant et après cinq secousses simples, à 0 degrees C, chez le sartorius de Rana temporaria, intoxiqué par l'acide monoiodoacétique. Arch Int Physiol Biochim. 1965 Mar;73(2):241–259. doi: 10.3109/13813456509084250. [DOI] [PubMed] [Google Scholar]
- Taylor E. W., Lymn R. W., Moll G. Myosin-product complex and its effect on the steady-state rate of nucleoside triphosphate hydrolysis. Biochemistry. 1970 Jul 21;9(15):2984–2991. doi: 10.1021/bi00817a008. [DOI] [PubMed] [Google Scholar]
- WAHLER B. E., WOLLENBERGER A. Zur Bestimmung des Orthophosphats neben säure-molybdat-labilen Phosphorsäureverbindungen. Biochem Z. 1958;329(6):508–520. [PubMed] [Google Scholar]
- WOLEDGE R. C. Heat production and energy liberation in the early part of a muscular contraction. J Physiol. 1963 Apr;166:211–224. doi: 10.1113/jphysiol.1963.sp007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLEDGE R. C. The thermoelastic effect of change of tension in active muscle. J Physiol. 1961 Jan;155:187–208. doi: 10.1113/jphysiol.1961.sp006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. H., Woledge R. C. Heat production and chemical change in tortoise muscle. J Physiol. 1970 Feb;206(2):457–469. doi: 10.1113/jphysiol.1970.sp009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizsäcker V. Myothermic experiments in salt-solutions in relation to the various stages of a muscular contraction. J Physiol. 1914 Sep 8;48(5):396–427. doi: 10.1113/jphysiol.1914.sp001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie D. R. Heat work and phosphorylcreatine break-down in muscle. J Physiol. 1968 Mar;195(1):157–183. doi: 10.1113/jphysiol.1968.sp008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. The intracellular site of calcium activaton of contraction in frog skeletal muscle. J Gen Physiol. 1970 Jan;55(1):77–88. doi: 10.1085/jgp.55.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]