Abstract
1. The excitability of synaptic structures in the cuneate nucleus was studied in eighteen decerebrate, unanaesthetized cats during acute changes in inspired PCO2.
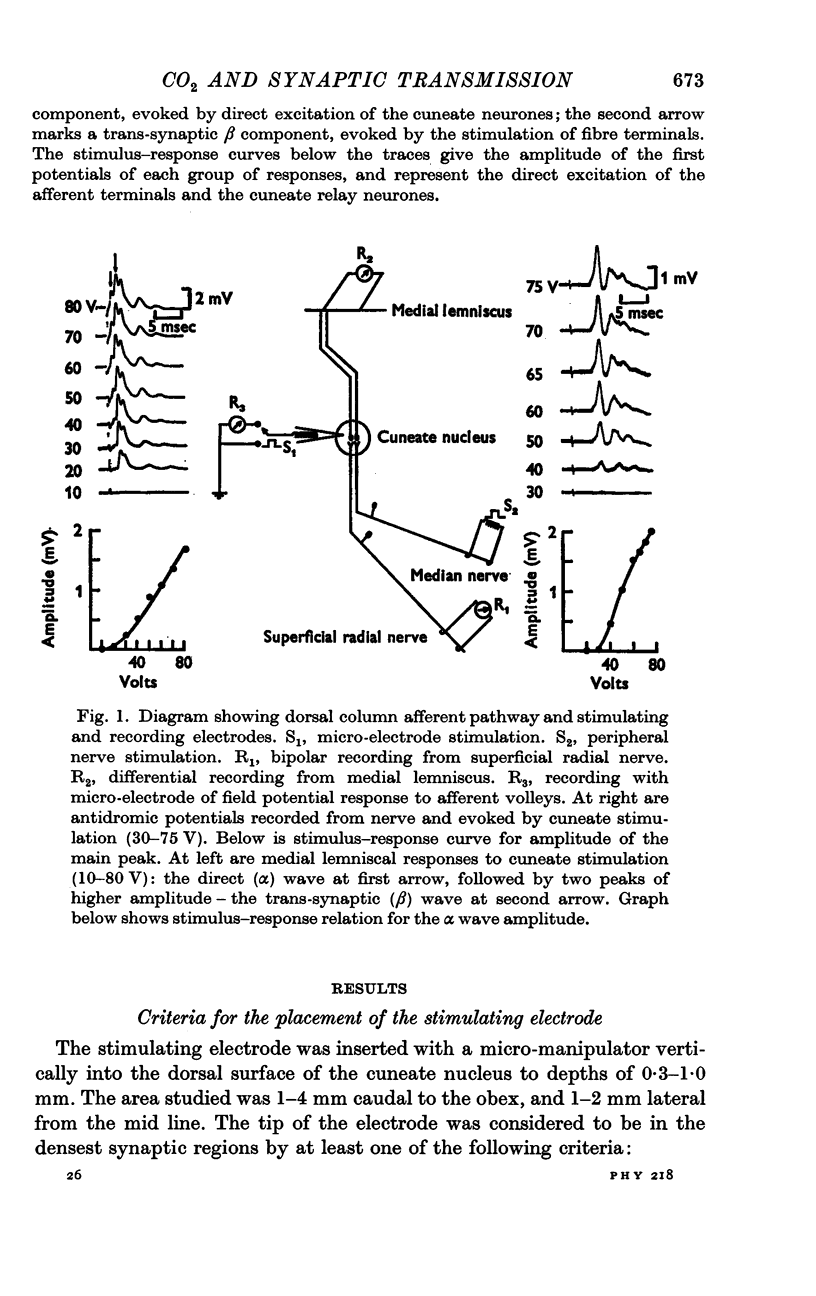
2. Micro-electrode stimulation in the caudal half of the cuneate nucleus evoked antidromic and orthodromic responses which were recorded simultaneously from a forelimb nerve and from the medial lemniscus at the transected mid-brain surface.
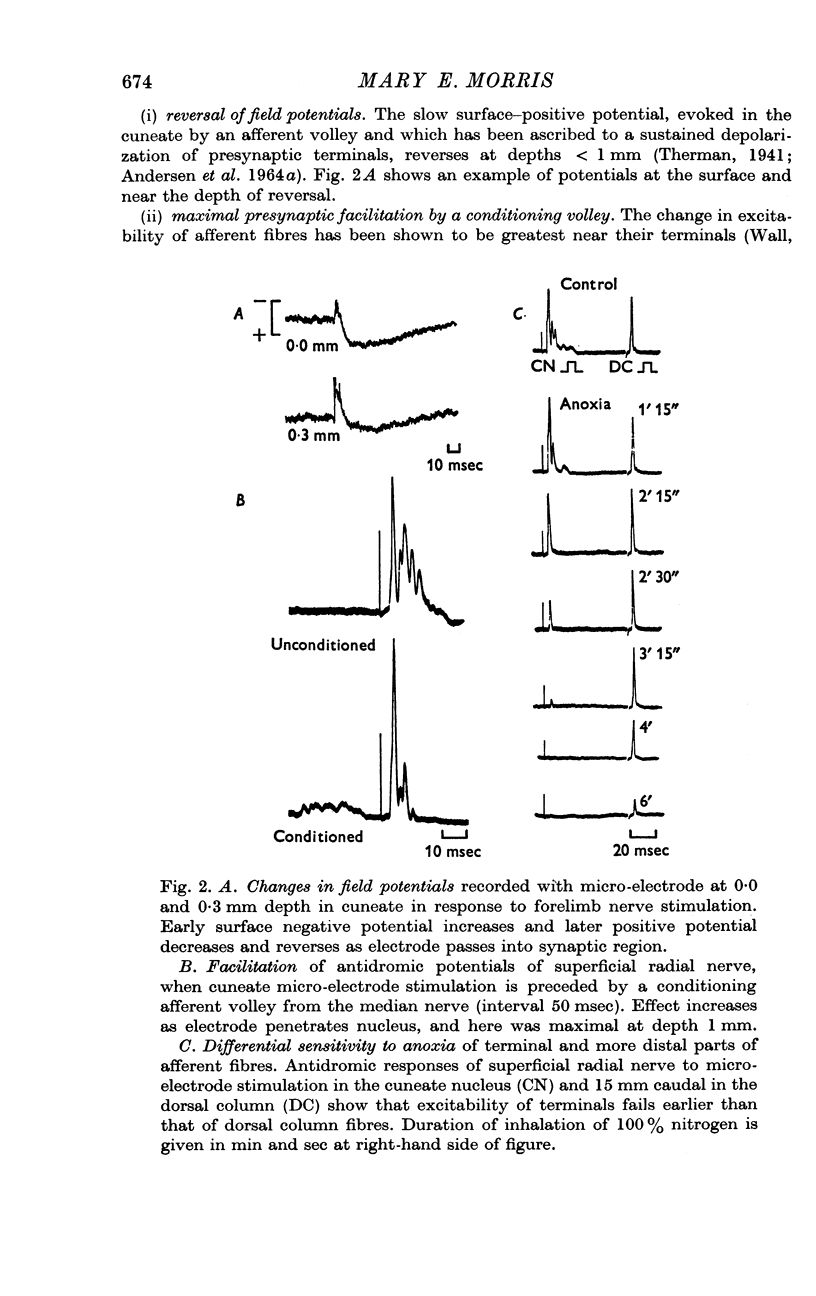
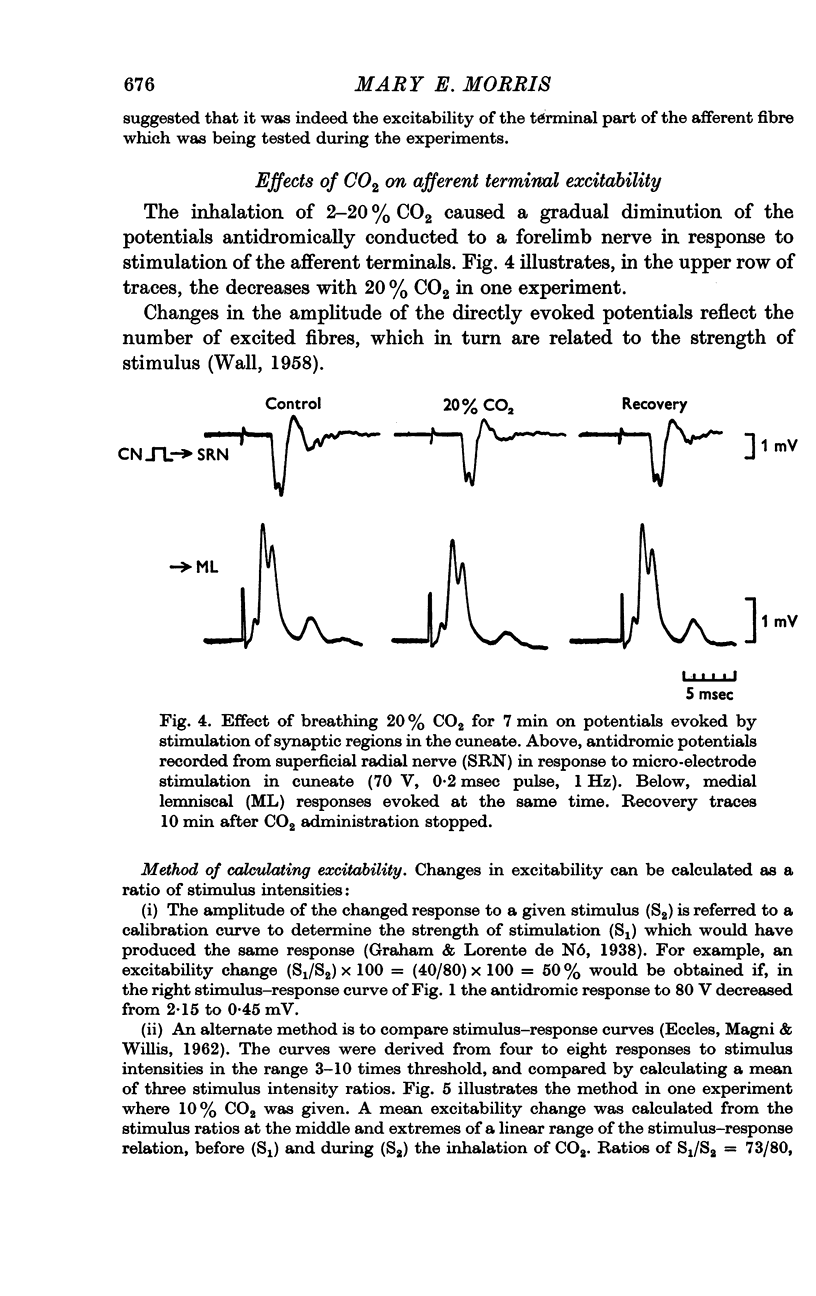
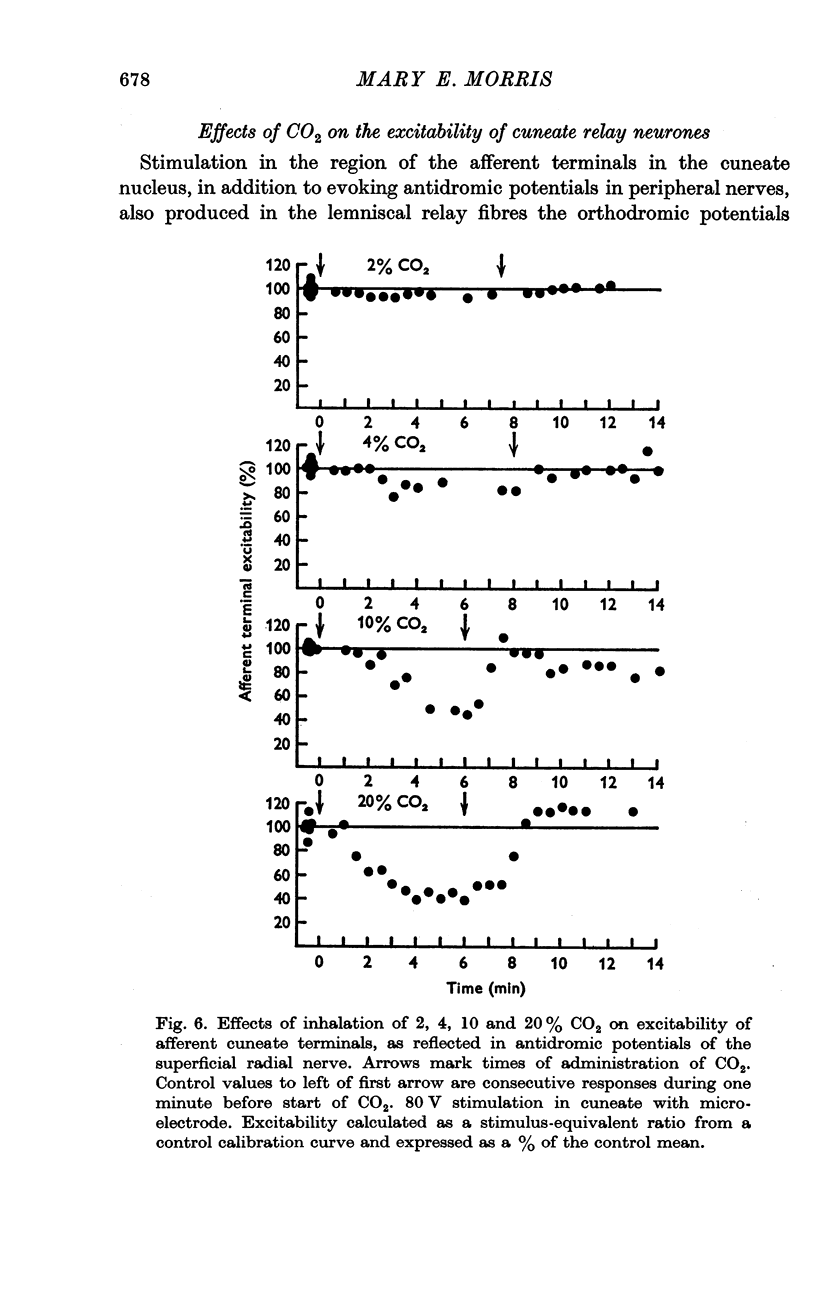
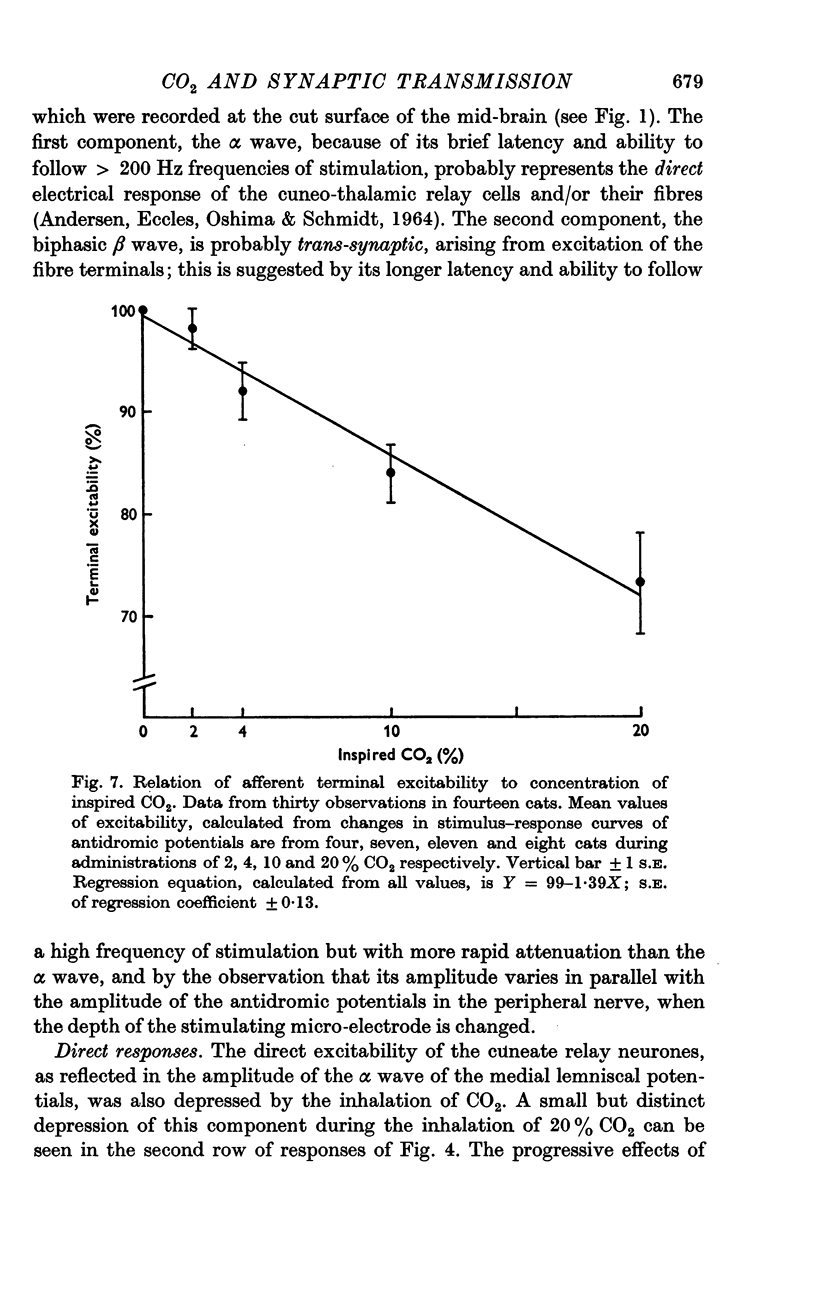
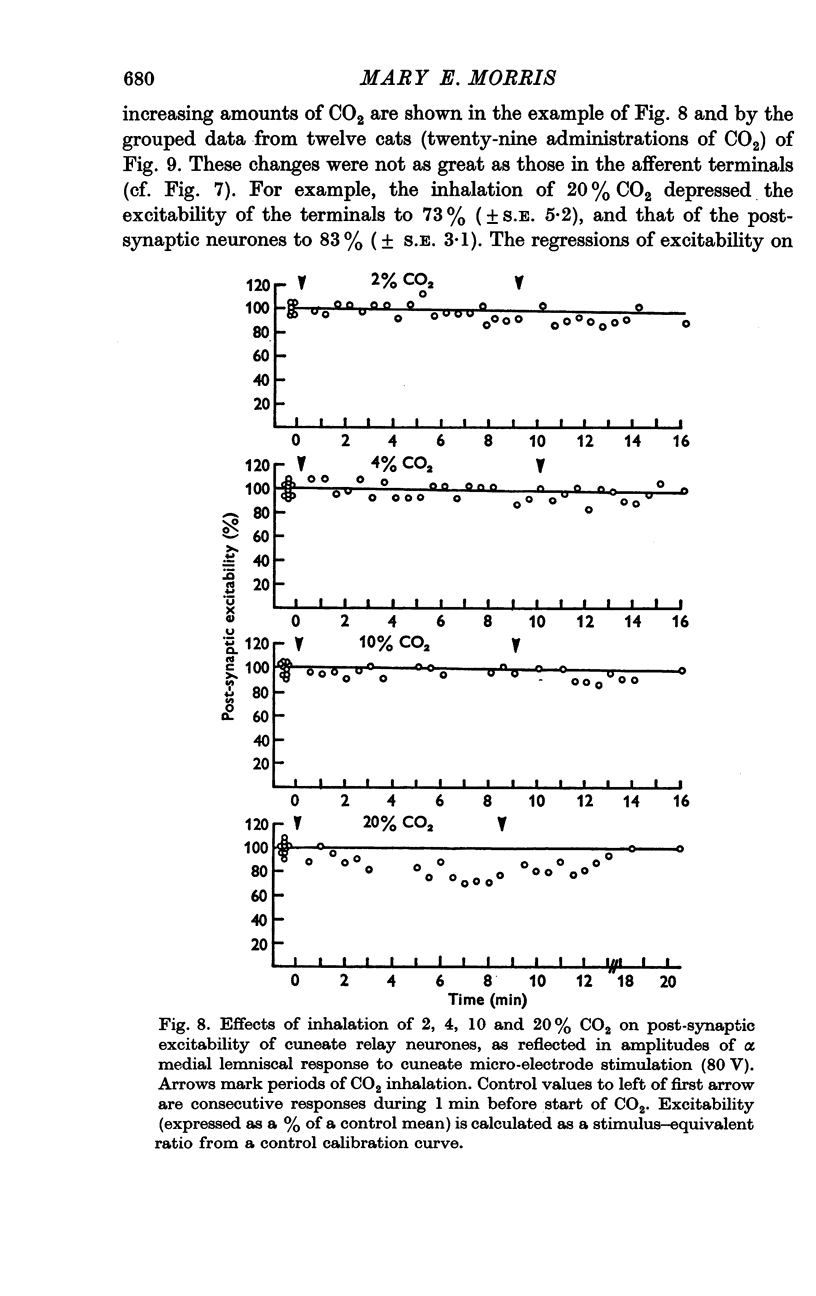
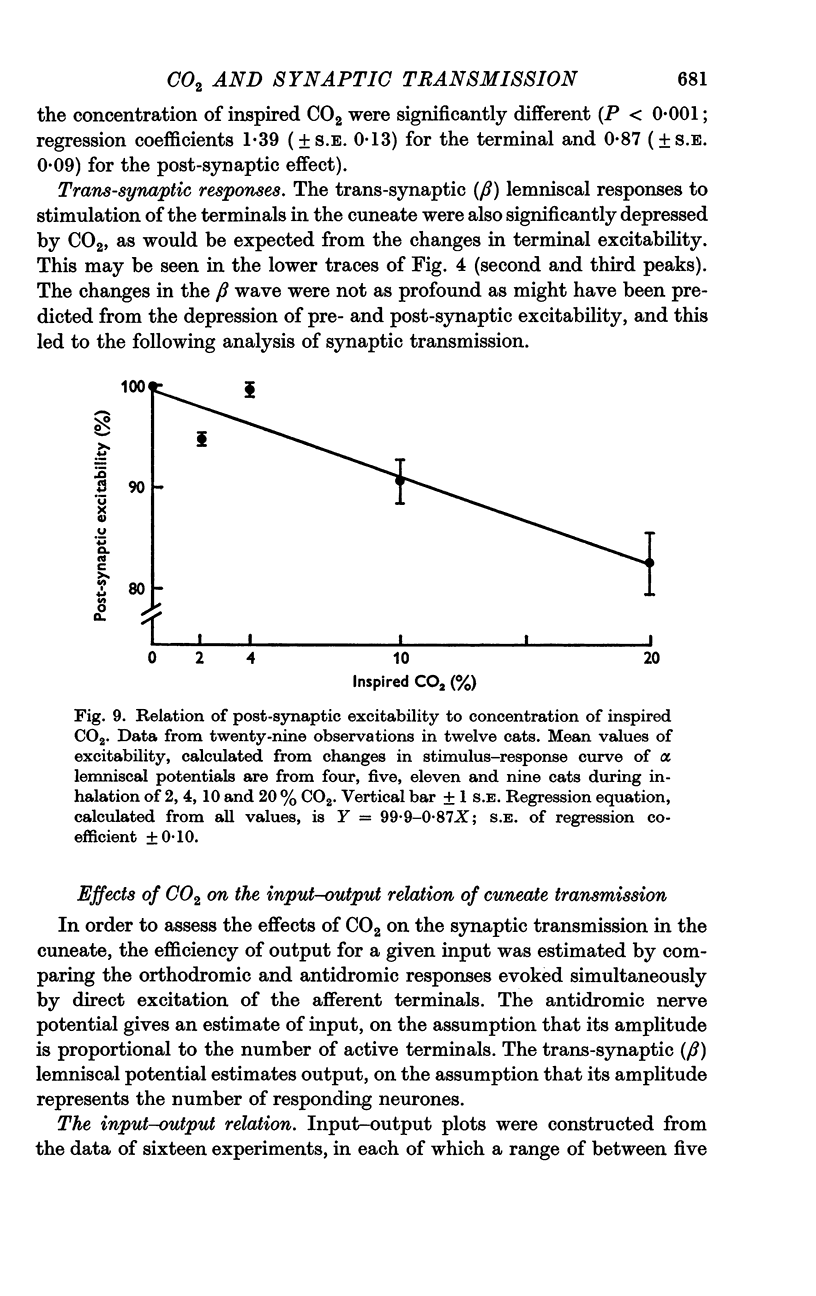
3. Increases in the concentration of inspired CO2 (2-20%) progressively decreased the direct excitability of both the afferent fibre terminals (reflected in the antidromic potentials) and the cuneate relay neurones (reflected in the α wave of the orthodromic lemniscal response). Synaptically mediated responses, recorded as the β component of the lemniscal potentials, were also depressed.
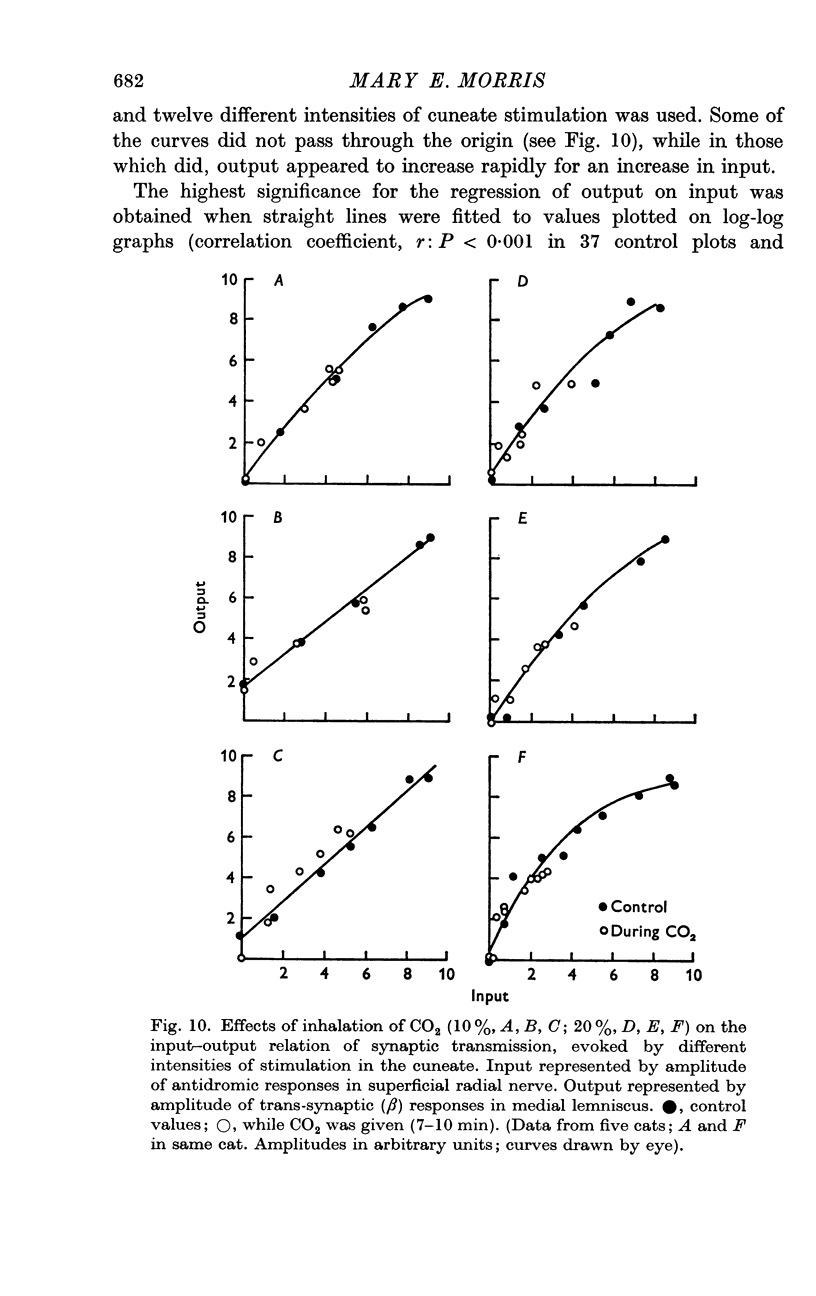
4. The relation between input and output at the cuneate was determined by plotting antidromic against trans-synaptic (β lemniscal) responses for different intensities of stimulation. The mean slope for logarithmic values of control potential amplitudes was 1·17 (± S.E. 0·13). It therefore appears that the transfer function for the cuneate is linear over a wide range.
5. In the majority of experiments the input—output relation was unchanged or increased by raising PCO2. It was concluded that the efficiency of synaptic transmission and release of transmitter appeared to be well maintained or possibly increased at individual active synapses during hypercarbia.
6. The depressant action of CO2 on afferent transmission can therefore be attributed largely to a block of impulse conduction in the primary afferent fibres.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. DEPOLARIZATION OF PRESYNAPTIC FIBERS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Jan;27:92–106. doi: 10.1152/jn.1964.27.1.92. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. SLOW POTENTIAL WAVES PRODUCED IN THE CUNEATE NUCLEUS BY CUTANEOUS VOLLEYS AND BY CORTICAL STIMULATION. J Neurophysiol. 1964 Jan;27:78–91. doi: 10.1152/jn.1964.27.1.78. [DOI] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Berman P. R. Mechanism of excitation of Aplysia neurons by carbon dioxide. J Gen Physiol. 1970 Nov;56(5):543–558. doi: 10.1085/jgp.56.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Sutton R. B., Walker J. L., Jr Increased chloride conductance as the proximate cause of hydrogen ion concentration effects in Aplysia neurons. J Gen Physiol. 1970 Nov;56(5):559–582. doi: 10.1085/jgp.56.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., NELSON T. E., Jr, SANCHEZ V. Mechanism of the increased acetylcholine sensitivity of skeletal muscle in low pH solutions. J Cell Comp Physiol. 1962 Feb;59:35–44. doi: 10.1002/jcp.1030590105. [DOI] [PubMed] [Google Scholar]
- EDISEN A. E. Effects of asphyxia and repetitive stimulation on intramedullary afferent fibers. Am J Physiol. 1957 Nov;191(2):225–232. doi: 10.1152/ajplegacy.1957.191.2.225. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., SEED W. A. An investigation of nucleus gracilis of the cat by antidromic stimulation. J Physiol. 1961 Mar;155:589–601. doi: 10.1113/jphysiol.1961.sp006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Effects of procaine, pentobarbital and halothane on synaptic transmission in the central nervous system. J Pharmacol Exp Ther. 1969 Oct;169(2):185–195. [PubMed] [Google Scholar]
- Galindo A., Krnjević K., Schwartz S. Micro-iontophoretic studies on neurones in the cuneate nucleus. J Physiol. 1967 Sep;192(2):359–377. doi: 10.1113/jphysiol.1967.sp008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Keller J. H., Hand P. J. Dorsal root projections to nucleus cuneatus of the cat. Brain Res. 1970 May 20;20(1):1–17. doi: 10.1016/0006-8993(70)90149-6. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. Input-output relation in a flexor reflex. J Gen Physiol. 1957 Nov 20;41(2):297–306. doi: 10.1085/jgp.41.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUSSIER J. J., RUSHTON W. A. H. The relation between the space constant and conduction velocity in nerve fibers of the A group from the frog's sciatic. J Physiol. 1951 Jul;114(3):399–409. doi: 10.1113/jphysiol.1951.sp004631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE A. K., MARK R. F. Synaptic linkage between afferent fibres of the cat's hind limb and ascending fibres in the dorsolateral funiculus. J Physiol. 1960 Sep;153:306–330. doi: 10.1113/jphysiol.1960.sp006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. E. The action of carbon dioxide on afferent transmission in the dorsal column-lemniscal system. J Physiol. 1971 Nov;218(3):651–669. doi: 10.1113/jphysiol.1971.sp009638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papajewski W., Klee M. R., Wagner A. The action of raised CO2 pressure on the excitability of spinal motorneurones. Electroencephalogr Clin Neurophysiol. 1969 Dec;27(6):618–618. [PubMed] [Google Scholar]
- RUDIN D. O., EISENMAN G. After-potential of spinal axons in vivo. J Gen Physiol. 1953 May;36(5):643–657. doi: 10.1085/jgp.36.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., HAGIWARA S. Capacity of muscle fiber membrane. Am J Physiol. 1957 Mar;188(3):423–429. doi: 10.1152/ajplegacy.1957.188.3.423. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]


