Abstract
1. The Ca movements in normal and `ghost' L cells have been examined; all measurements were made using 45Ca.
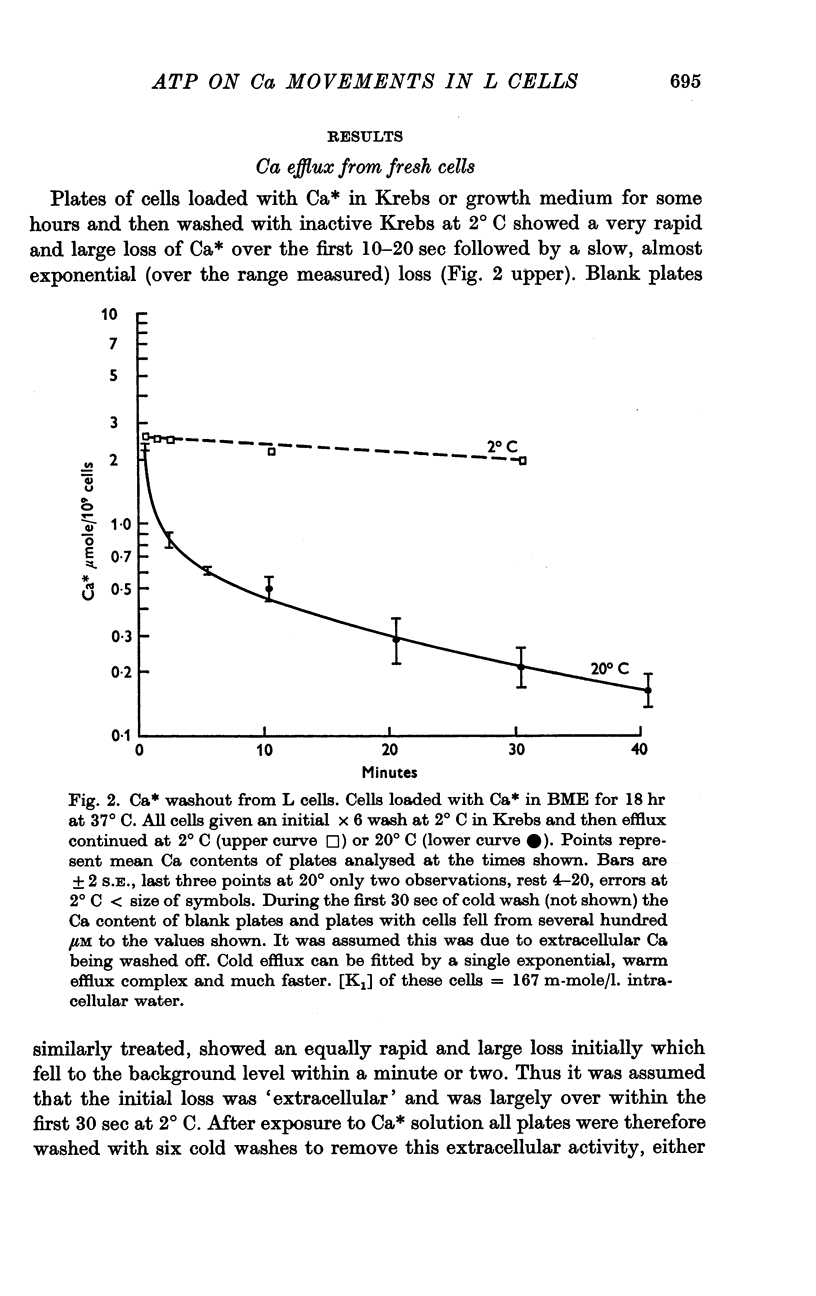
2. Normal cells have a Ca concentration of about 1 m-mole/l. of cell volume, and exchange Ca in a complex way but with great rapidity; the time taken for the initial Ca* content to fall to half was less than 2 min.
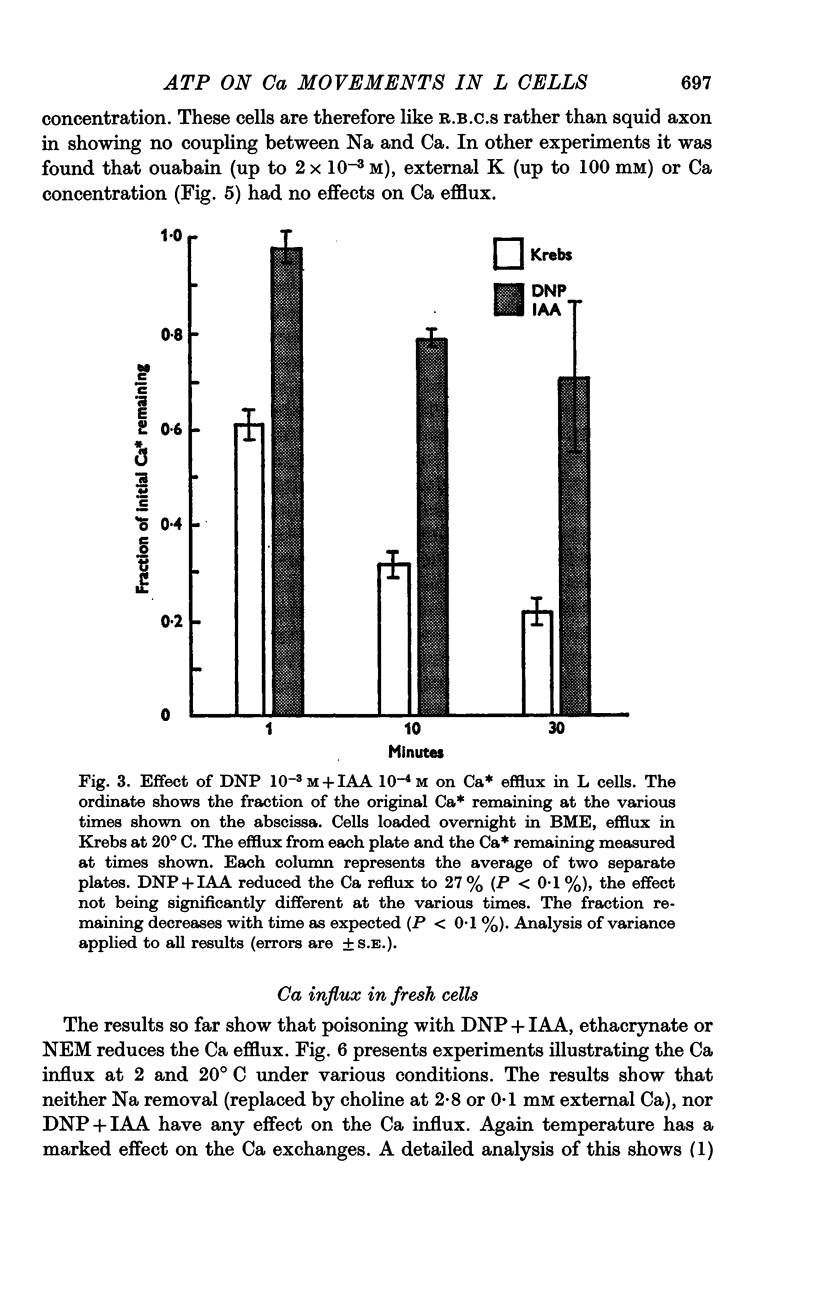
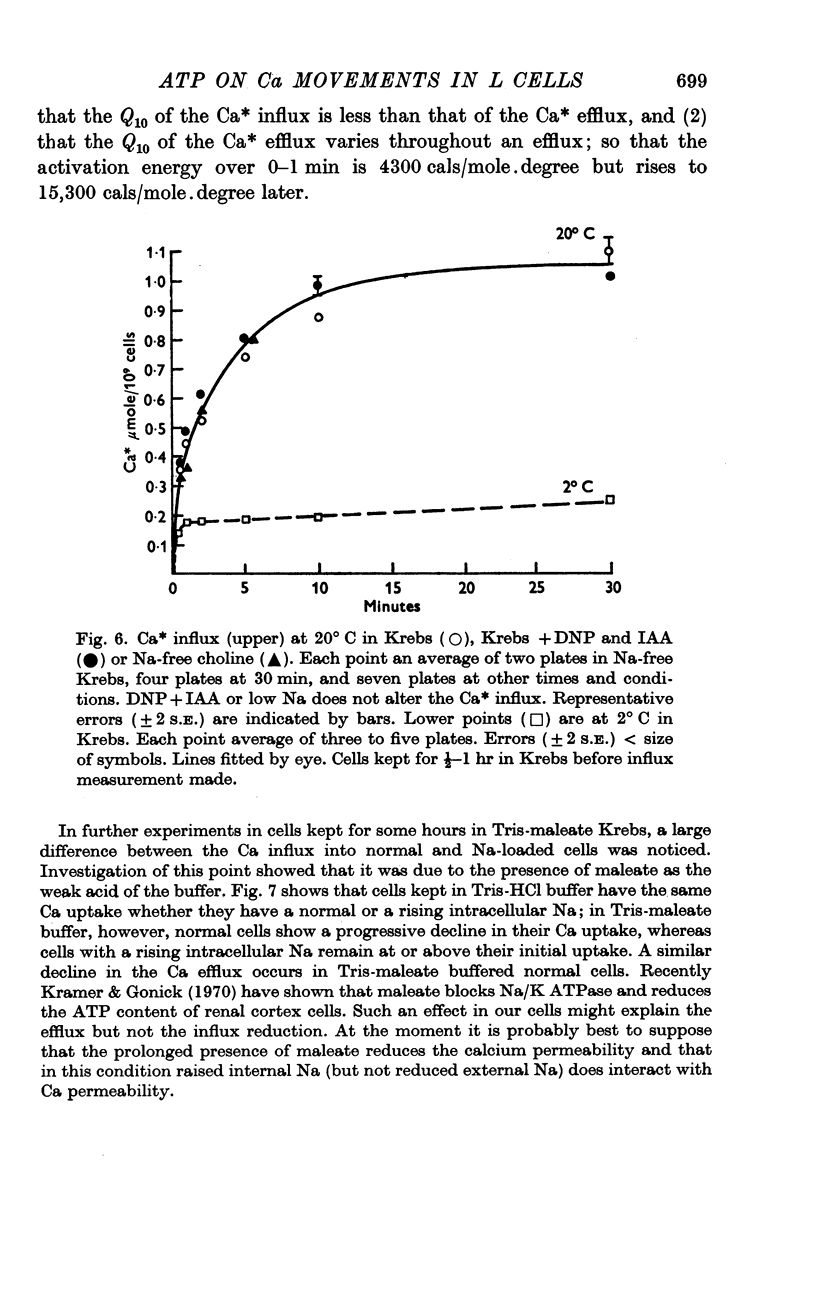
3. Poisoning normal cells with DNP 10-3 M + IAA 10-4 M causes a marked reduction in the Ca efflux, no change in Ca influx and an increase in total Ca.
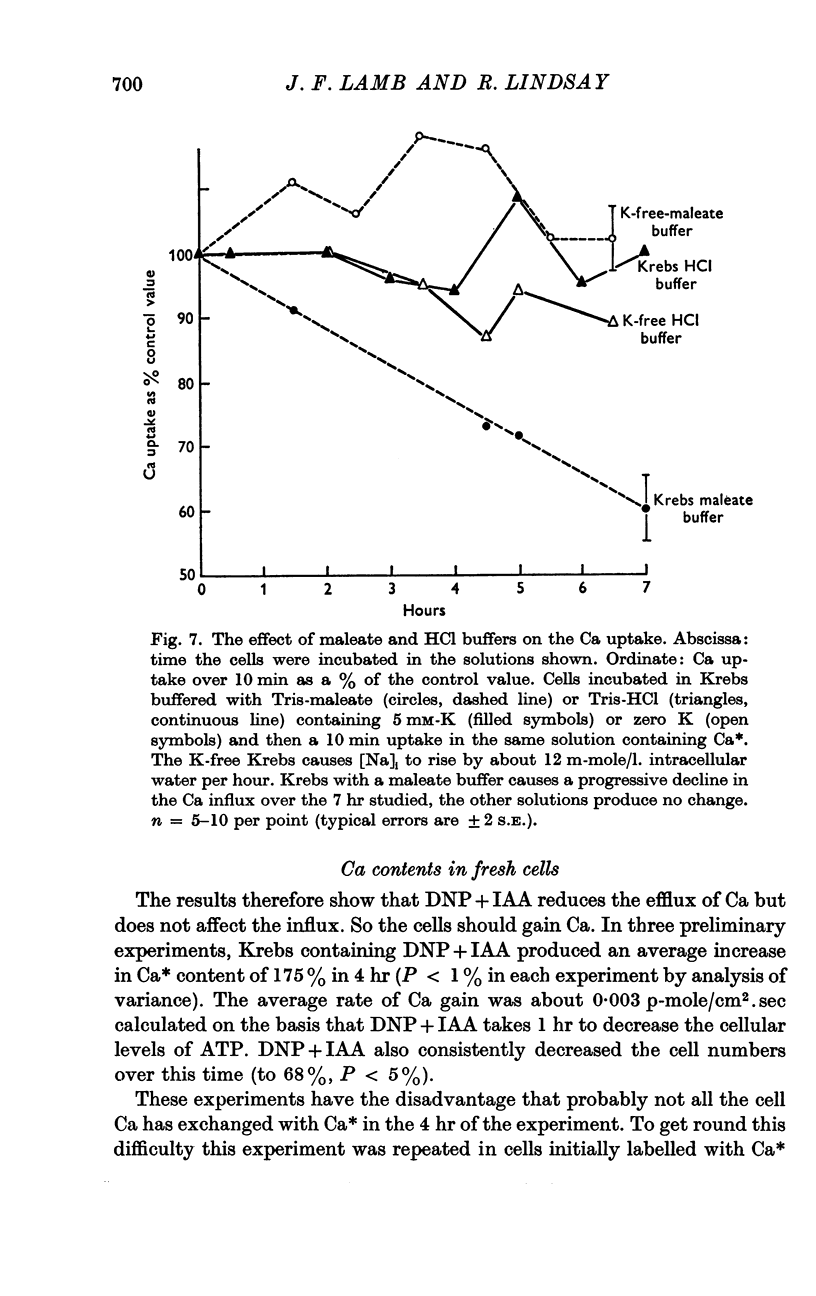
4. Variation in internal or external Na concentration does not alter the Ca fluxes or concentrations. Application of cyanide or ouabain and alteration of external K concentration had no effect on the Ca fluxes.
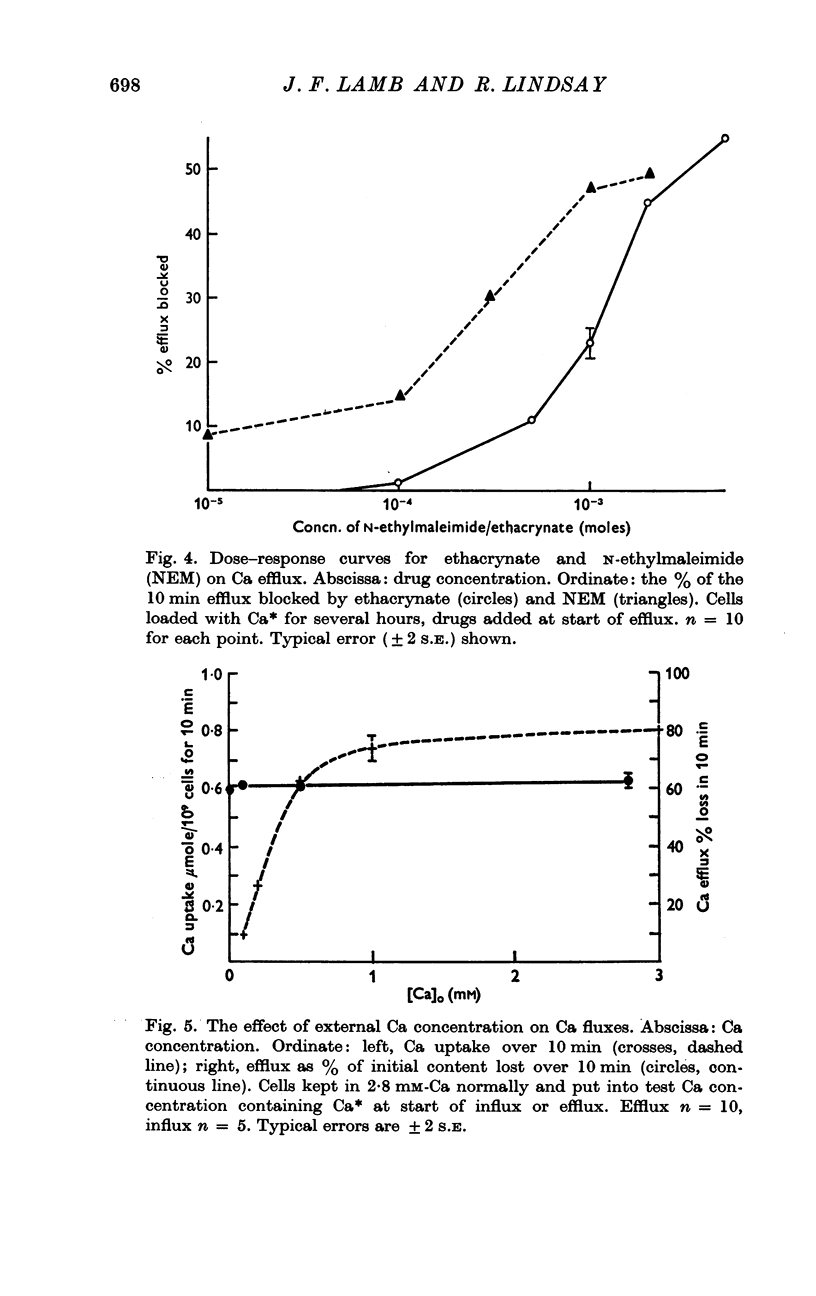
5. The sulphydryl reagents, ethacrynic acid and N-ethylmaleimide (NEM), have a rapid and marked effect on reducing the Ca efflux.
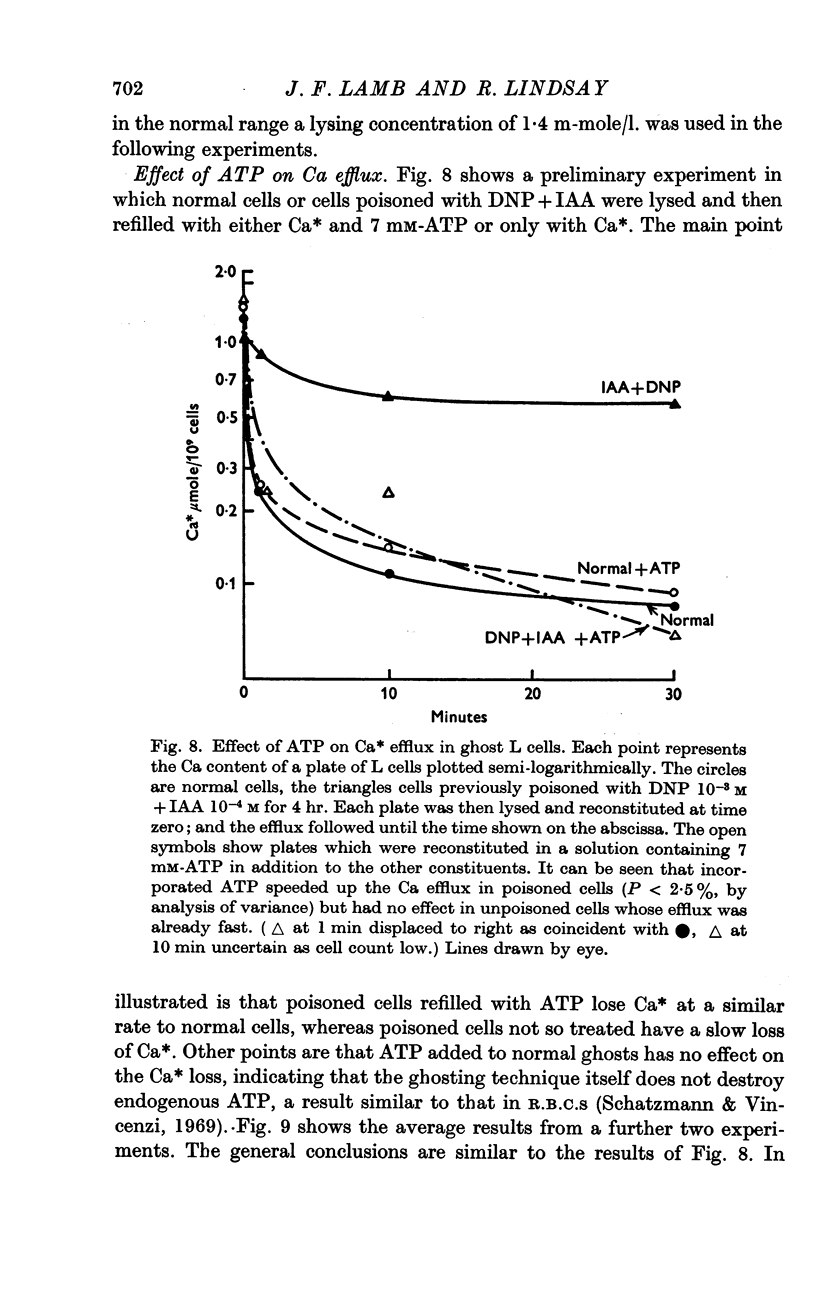
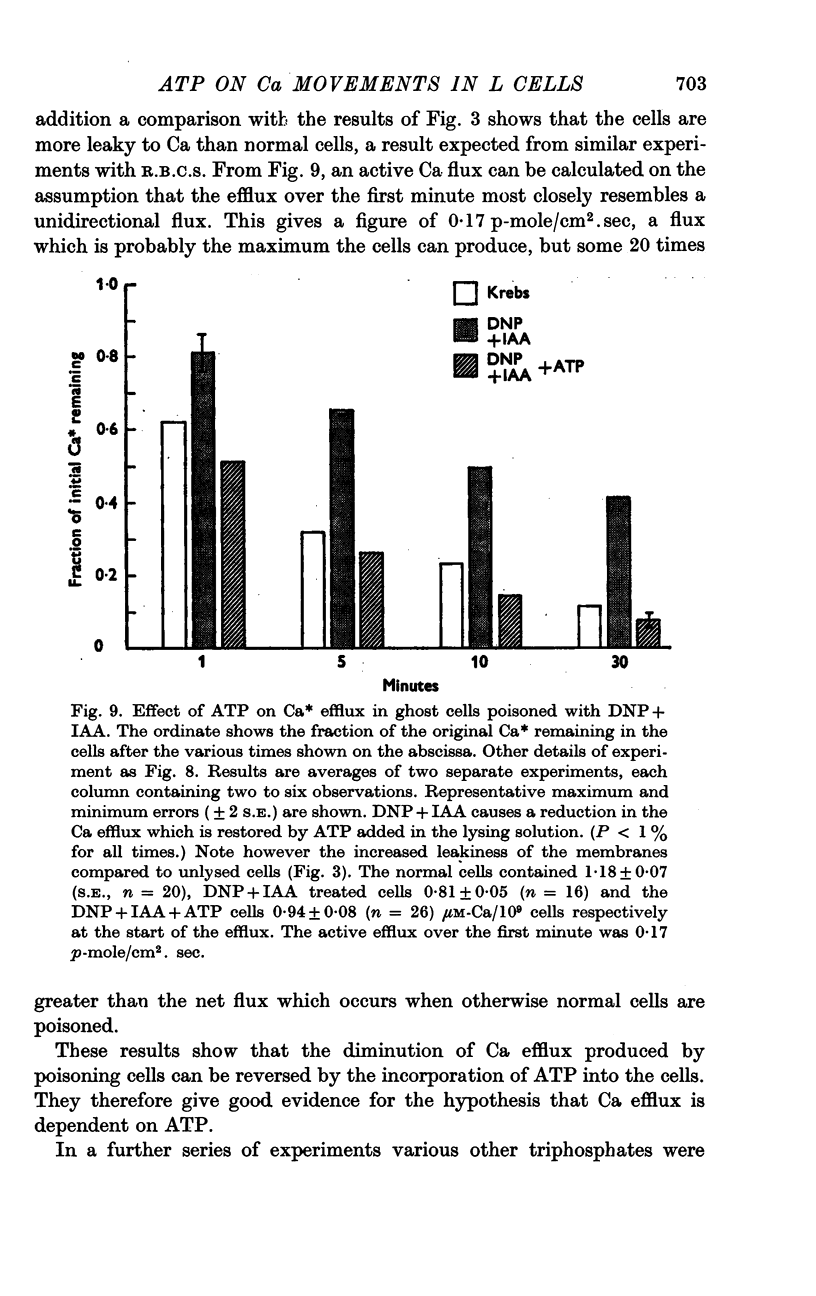
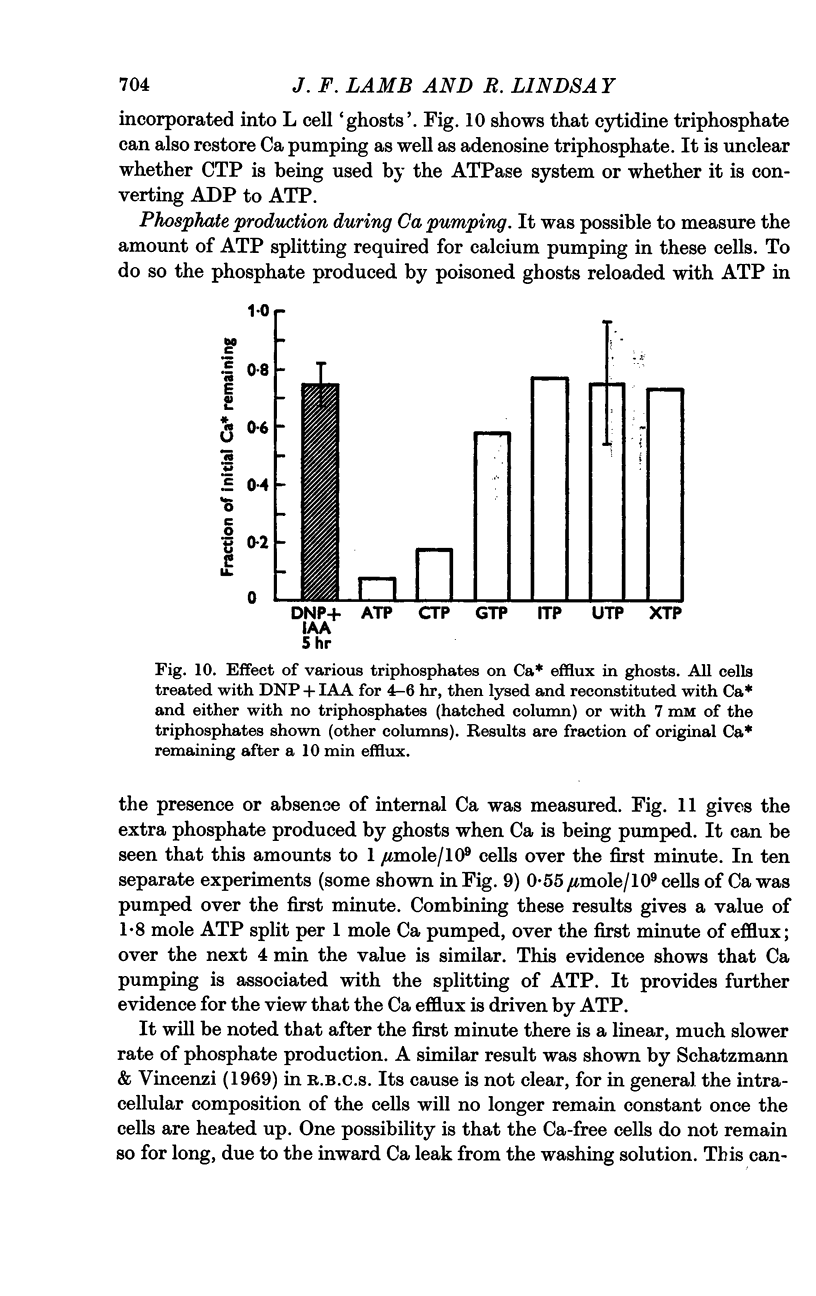
6. L cell ghosts previously poisoned with DNP+IAA have a low Ca efflux. When ATP or CTP is incorporated into such cells the Ca efflux becomes normal.
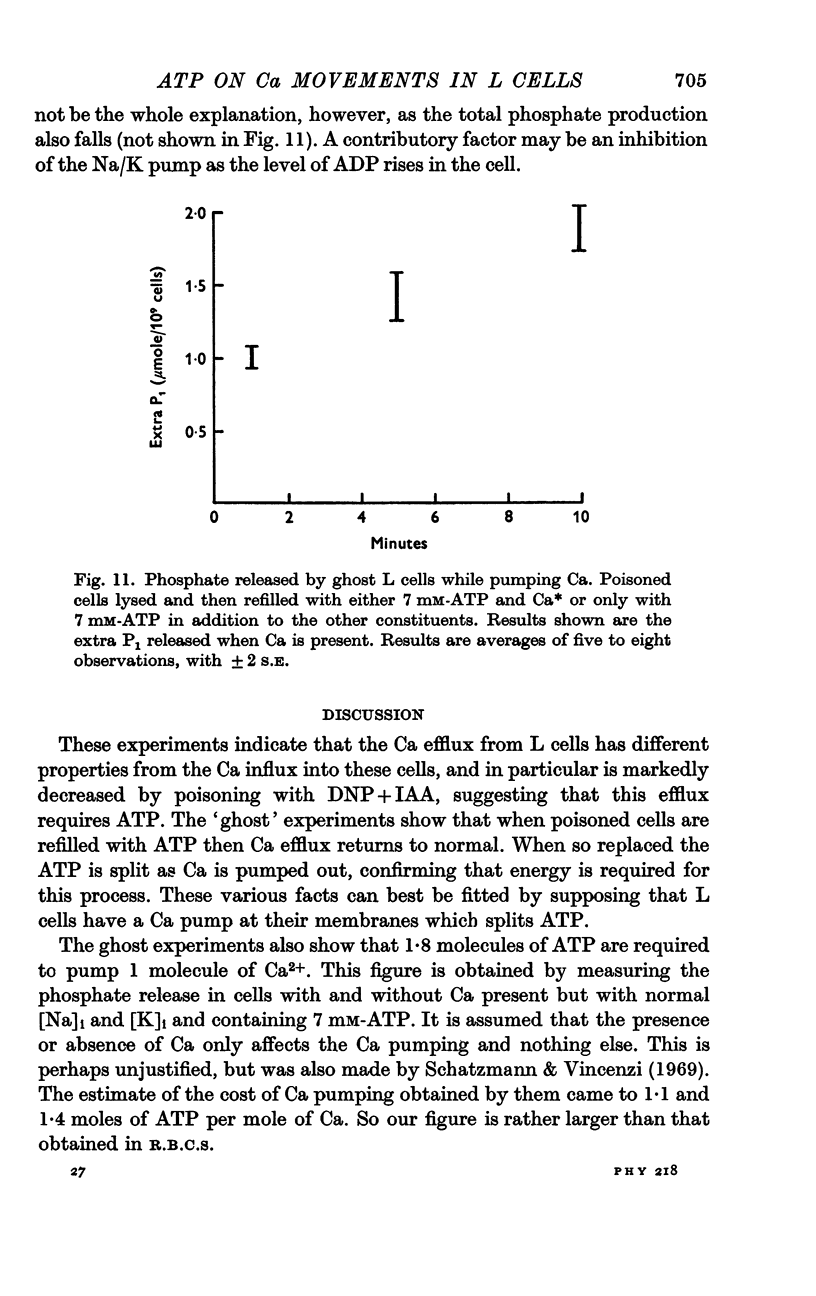
7. An extra amount of phosphate is produced by L cell ghosts when pumping Ca. This is equivalent to the splitting of 1·8 moles of ATP per mole of Ca pumped.
8. It is concluded that L cells have a Ca pump driven by ATP, and that Na has no effect on Ca movements in these cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. Studies on the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):286–294. doi: 10.1042/bj0320286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. I. Calcium influx. J Gen Physiol. 1969 Jan;53(1):43–56. doi: 10.1085/jgp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. II. Calcium efflux. J Gen Physiol. 1969 Jan;53(1):57–69. doi: 10.1085/jgp.53.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANES B. S., PAUL J. Environmental factors influencing respiration of strain L cells. Exp Cell Res. 1961 Aug;24:344–355. doi: 10.1016/0014-4827(61)90437-2. [DOI] [PubMed] [Google Scholar]
- Davis P. W. Inhibition of renal Na + ,K + -activated adenosine triphosphatase activity by ethacrynic acid. Biochem Pharmacol. 1970 Jun;19(6):1983–1989. doi: 10.1016/0006-2952(70)90294-7. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Gonick H. C. Experimental Fanconi syndrome. I. Effect of maleic acid on renal cortical Na-K-ATPase activity and ATP levels. J Lab Clin Med. 1970 Nov;76(5):799–808. [PubMed] [Google Scholar]
- Lamb J. F., MacKinnon M. G. Effect of ouabain and metabolic inhibitors on the Na and K movements and nucleotide contents of L cells. J Physiol. 1971 Mar;213(3):665–682. doi: 10.1113/jphysiol.1971.sp009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., MacKinnon M. G. The membrane potential and permeabilities of the L cell membrane to Na, K and chloride. J Physiol. 1971 Mar;213(3):683–689. doi: 10.1113/jphysiol.1971.sp009408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]


