Abstract
1. Ganglion cells in the inferior mesenteric ganglion (IMG) and the pelvic plexus of the guinea-pig were studied using intracellular micro-electrodes.
2. Ganglion cells had resting membrane potentials of 55-65 mV. Threshold for initiation of an action potential ranged from 10 to 20 mV depolarization. Action potentials often exceeded 100 mV in amplitude and were followed by an after-hyperpolarization of up to 20 mV.
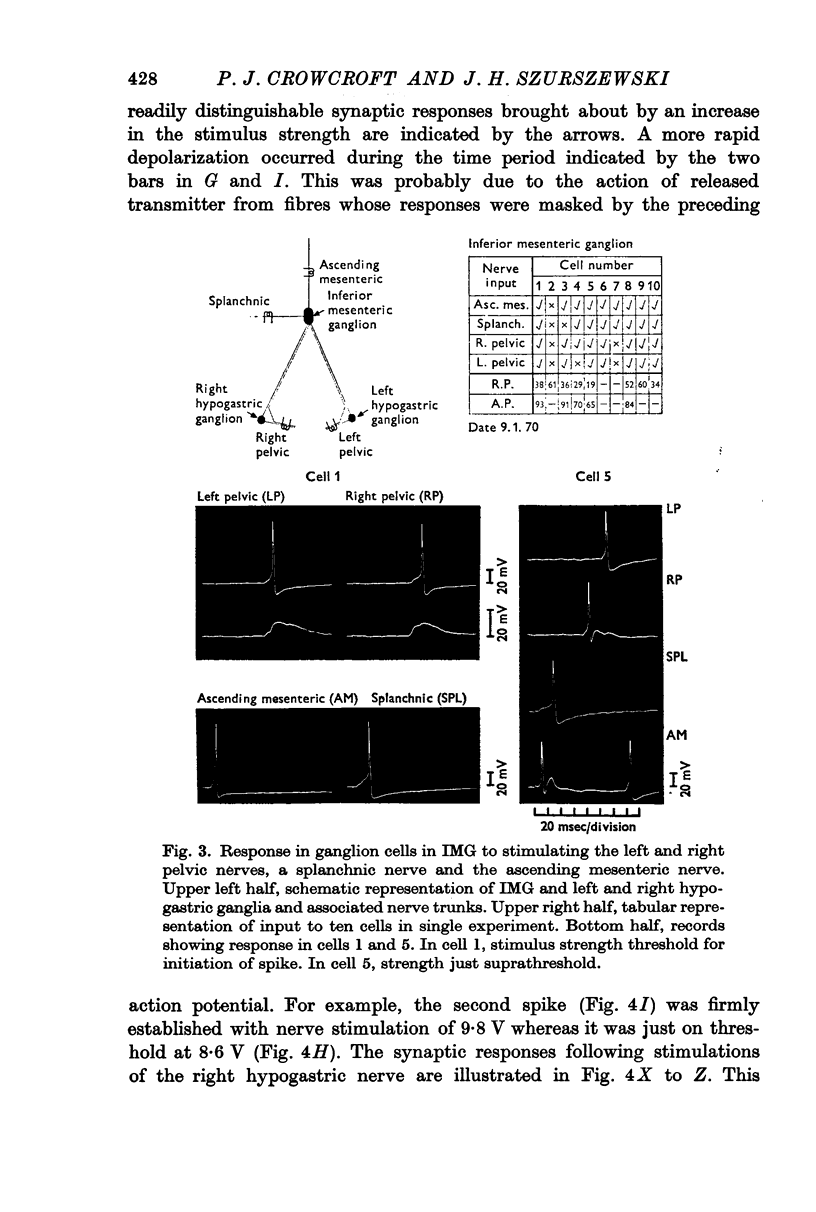
3. Synaptic responses were recorded from cells in the IMG in response to stimulation of the right and left hypogastric nerves, ascending mesenteric, inferior splanchnic and colonic nerves. It has been established that more than forty preganglionic fibres converge on any one cell. Preganglionic fibres to the IMG were also observed in the pelvic nerves.
4. In contrast to the IMG, ganglion cells in the pelvic plexus received up to ten preganglionic fibres.
5. Ganglion cells responded to supramaximal preganglionic stimulation with up to four action potentials.
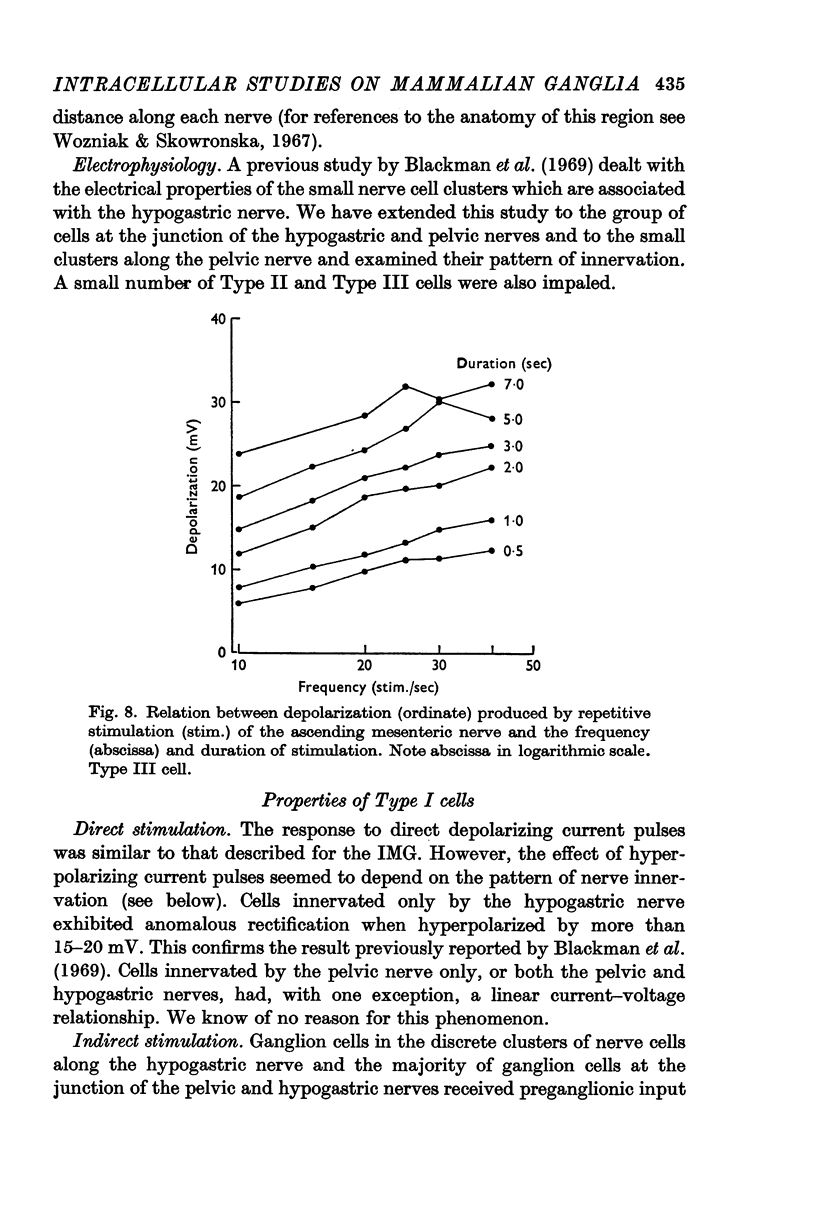
6. In the IMG, action potentials in response to synaptic action were followed by a prolonged period of hyperpolarization (after-hyperpolarization) and a later phase of prolonged depolarization (after-depolarization). The time course of these after potentials depended on the pattern of firing of action potentials during the period of stimulation. In the presence of dihydro-β-erythroidine, or if synaptic action was insufficient to evoke action potentials, only the after-depolarization was observed.
7. Other cells were impaled whose properties differed from those described above. In one group of cells the resting membrane potentials were higher (up to 85 mV), input resistances lower and the threshold for initiation of an action potential was higher. The other group were inexcitable, had high resting membrane potentials (up to 85 mV), low input resistances and underwent a slow depolarization in response to repetitive stimulation of preganglionic fibres.
8. This study indicates that marked convergence of presynaptic fibres occurs on to ganglion cells of the IMG. The ganglion cells in the pelvic plexus receive a relatively small number of fibres, many of which exert intense synaptic activity ensuring a direct connexion to the central nervous system.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nicholls J. G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):555–569. doi: 10.1113/jphysiol.1969.sp008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Gerschenfeld H. M. Some physiological properties of identified mammalian neuroglial cells. J Physiol. 1969 Jul;203(1):211–222. doi: 10.1113/jphysiol.1969.sp008860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Woodward J. K. Intracellular recording from mammalian superior cervical ganglion in situ. J Physiol. 1968 Nov;199(1):189–203. doi: 10.1113/jphysiol.1968.sp008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Muir T. C., Szurszewski J. H., Yonemura K. Effect of iontophoretic application of cholinergic agonists and their antagonists to guinea-pig pelvic ganglia. Br J Pharmacol. 1971 Jan;41(1):26–40. doi: 10.1111/j.1476-5381.1971.tb09932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOB C., LUNDBERG A. Reflex excitation of cells in the inferior mesenteric ganglion on stimulation of the hypogastric nerve. Acta Physiol Scand. 1952;26(4):366–382. doi: 10.1111/j.1748-1716.1952.tb00918.x. [DOI] [PubMed] [Google Scholar]
- KUNTZ A., JACOBS M. W. Components of periarterial extensions of celiac and mesenteric plexuses. Anat Rec. 1955 Dec;123(4):509–520. doi: 10.1002/ar.1091230409. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- Libet B., Tosaka T. Dopamine as a synaptic transmitter and modulator in sympathetic ganglia: a different mode of synaptic action. Proc Natl Acad Sci U S A. 1970 Oct;67(2):667–673. doi: 10.1073/pnas.67.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Tosaka T. Slow inhibitory and excitatory postsynaptic responses in single cells of mammalian sympathetic ganglia. J Neurophysiol. 1969 Jan;32(1):43–50. doi: 10.1152/jn.1969.32.1.43. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Raisman G. The ultrastructure and somatic efferent synapses of small granule-containing cells in the superior cervical ganglion. J Anat. 1969 Sep;105(Pt 2):255–282. [PMC free article] [PubMed] [Google Scholar]
- McLENNAN H., PASCOE J. E. The origin of certain non-medullated nerve fibres which form synapses in the inferior mesenteric ganglion of the rabbit. J Physiol. 1954 Apr 28;124(1):145–156. doi: 10.1113/jphysiol.1954.sp005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSCARSSON O. On the functional organization of the two presynaptic systems to the colonic nerve neurons of the inferior mesenteric ganglion in the cat. Acta Physiol Scand. 1955 Dec 31;35(2):153–166. doi: 10.1111/j.1748-1716.1955.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Evoked sustained focal potentials and membrane potential of neurons and of unresponsive cells of the spinal cord. J Neurophysiol. 1970 Jul;33(4):562–582. doi: 10.1152/jn.1970.33.4.562. [DOI] [PubMed] [Google Scholar]
- TAKESHIGE C., VOLLE R. L. Bimodal response of sympathetic ganglia to acetylcholine following eserine or repetitive preganglionic stimulation. J Pharmacol Exp Ther. 1962 Oct;138:66–73. [PubMed] [Google Scholar]
- Van Orden L. S., 3rd, Burke J. P., Geyer M., Lodoen F. V. Localization of depletion-sensitive and depletion-resistant norepinephrine storage sites in autonomic ganglia. J Pharmacol Exp Ther. 1970 Jul;174(1):56–71. [PubMed] [Google Scholar]
- Woźniak W., Skowrońska U. Comparative anatomy of pelvic plexus in cat, dog, rabbit, macaque and man. Anat Anz. 1967;120(5):457–473. [PubMed] [Google Scholar]


