Abstract
1. The properties of the receptive fields of simple cells in the cat striate cortex have been studied by preparing average response histograms both to moving slits of light of different width and to single light-dark edges or contours.
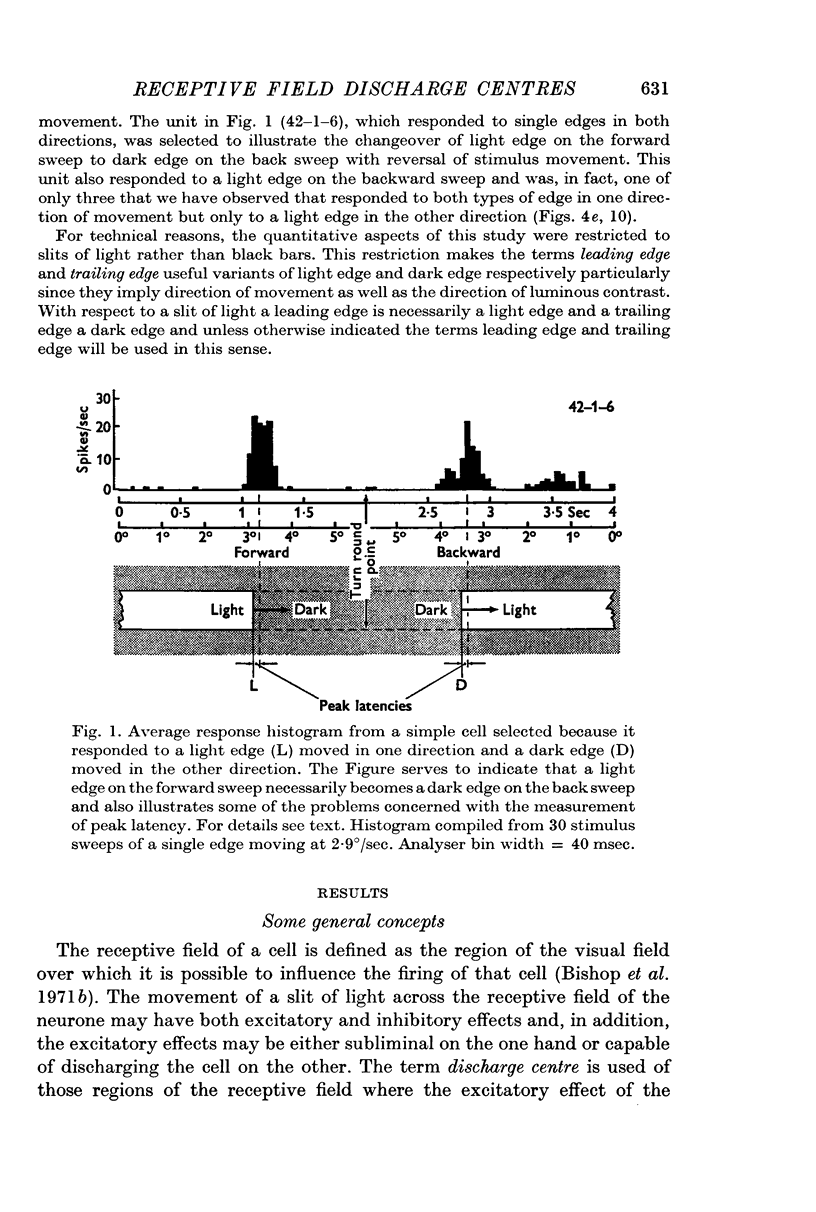
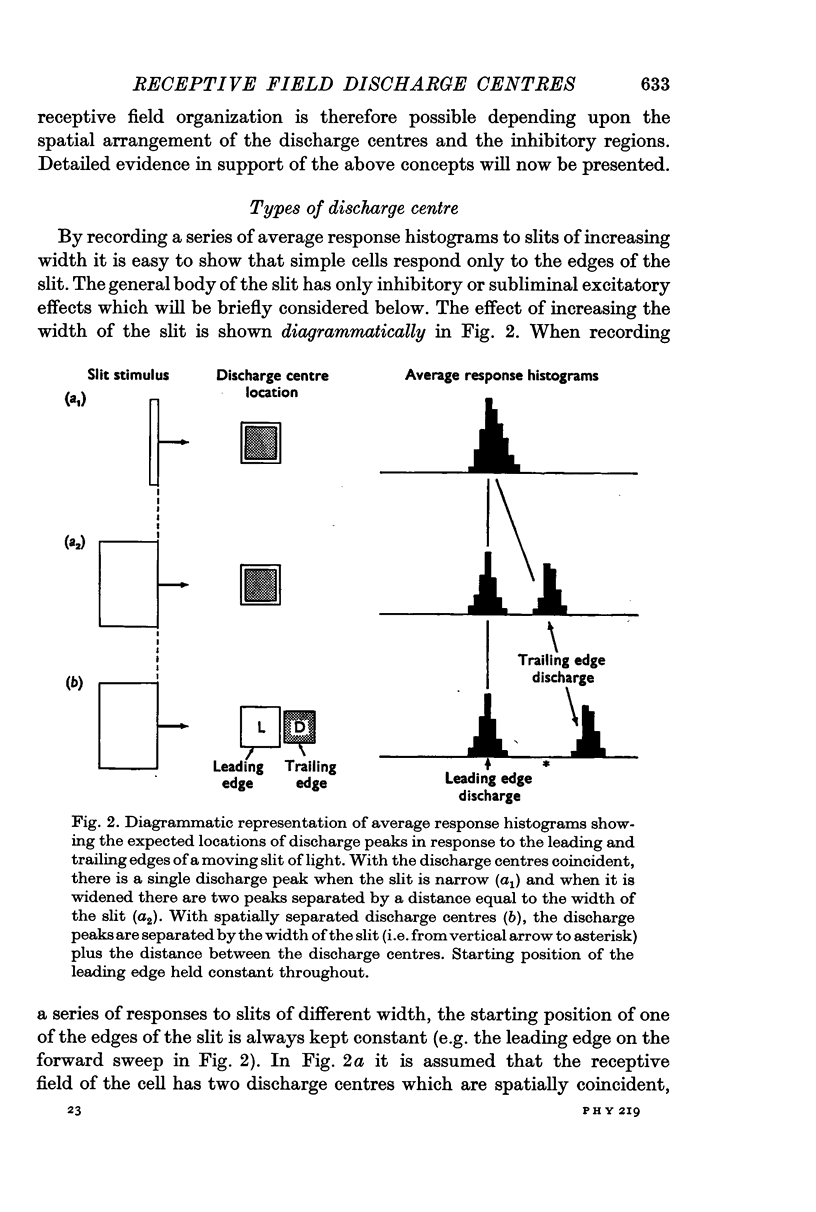
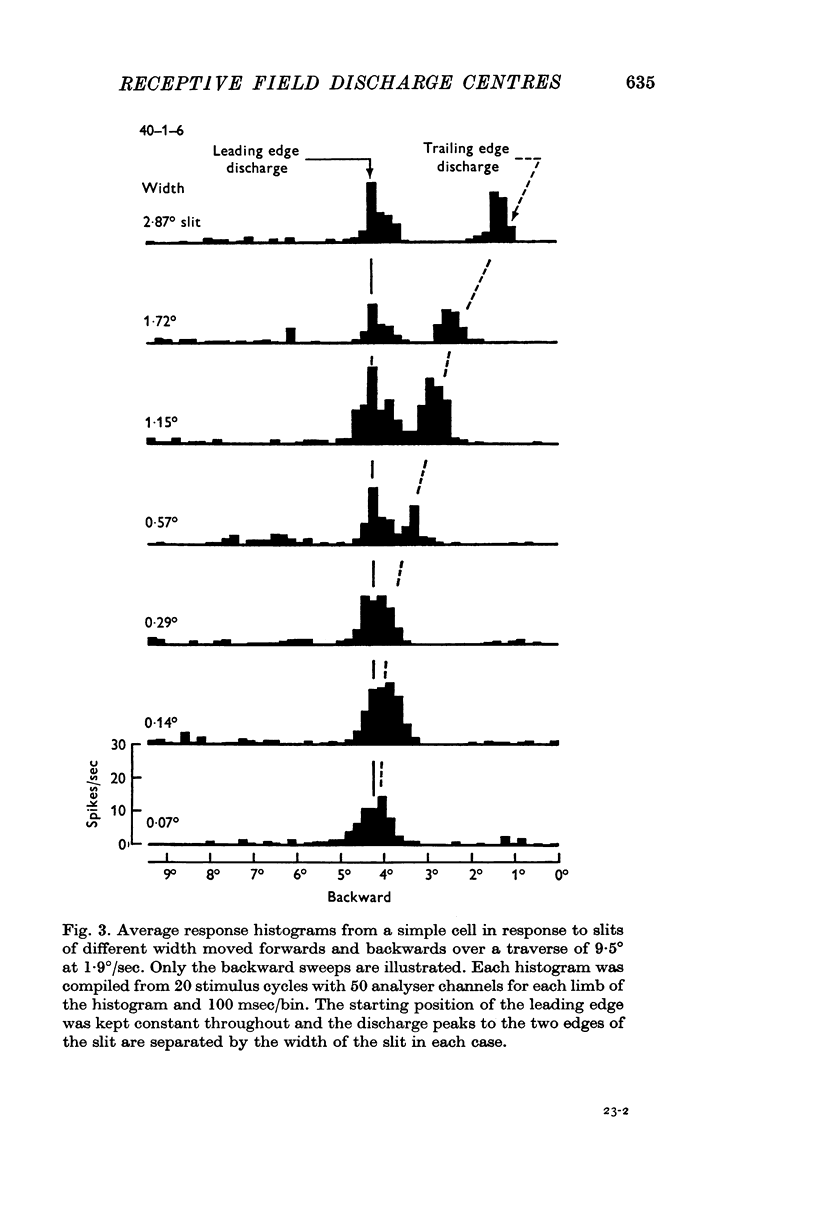
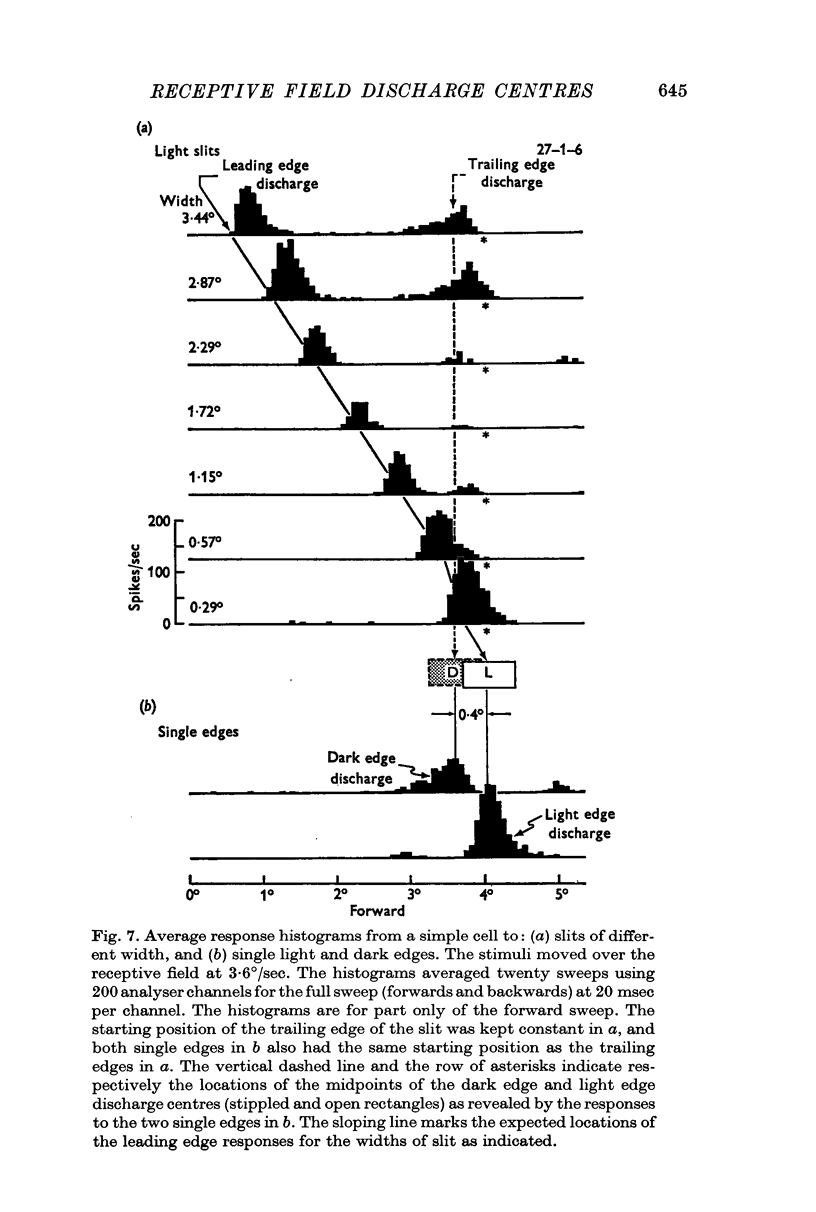
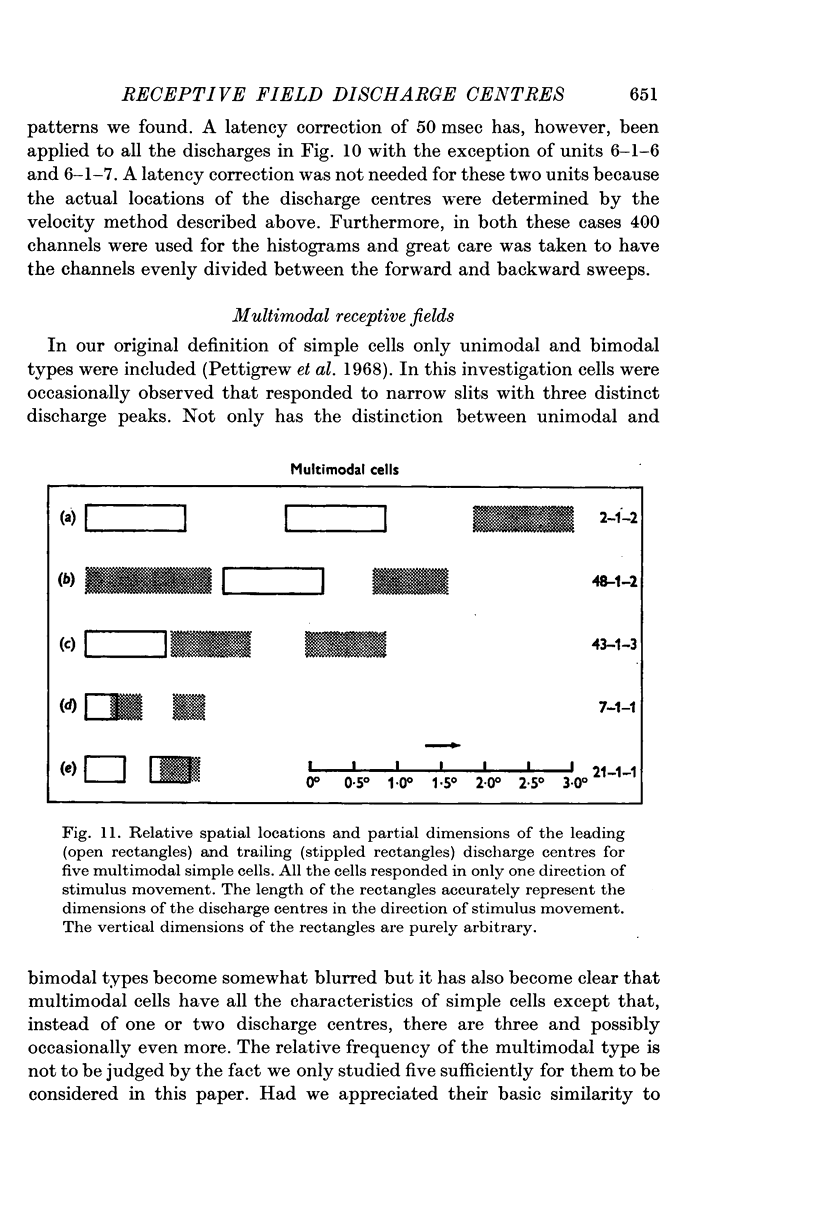
2. The movement of a narrow (< 0·3°) slit across the receptive field gives rise to average response histograms that are either unimodal, bimodal or multimodal. A slit of light has leading (light) and trailing (dark) edges. By increasing the width of the slit it was shown that a discharge peak in the histogram coincides with the passage of one or other of the two edges over a particular region (discharge centre) in the receptive field. Each edge has its own discharge centre which is fired when the edge has the correct orientation and direction of movement.
3. The discharge centres in forty-three simple cell receptive fields were located by using one or more of the following stimuli for each cell:
(i) slits of different width;
(ii) single light and dark edges;
(iii) a wide (3°) slit moved over a range of different velocities.
The same locations were obtained when all three procedures were used on the same cell.
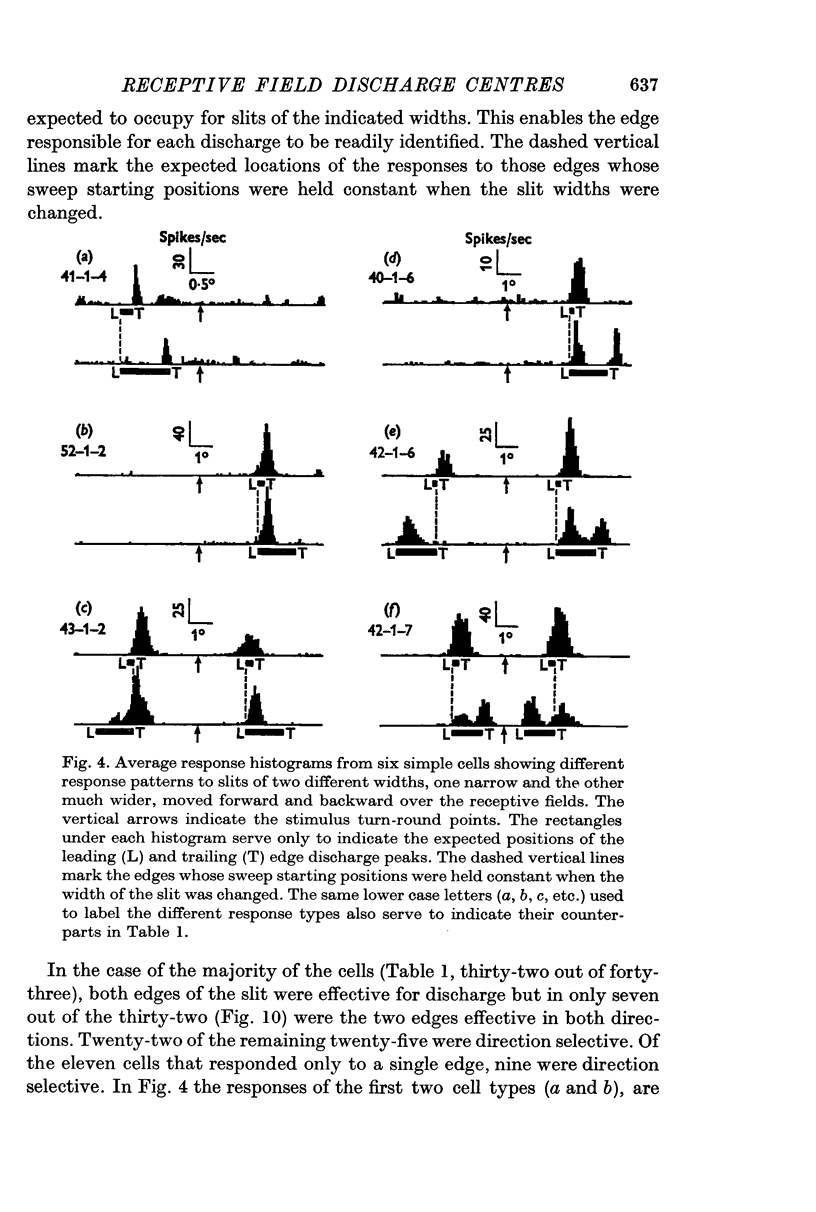
4. Most cells (79%) discharged to both edges though not necessarily in the same direction of movement. The majority (72%) fired in only one direction and most commonly (51%) the cells responded to both edges in this one direction. In only 16% of cells did both types of edge excite in both directions of movement. When the one type of edge, light or dark, was considered, 84% of the cells were direction selective and, for these cells, the other edge fired only in the same direction (51%), in both directions (7%), only in the opposite direction (5%) or not at all (21%).
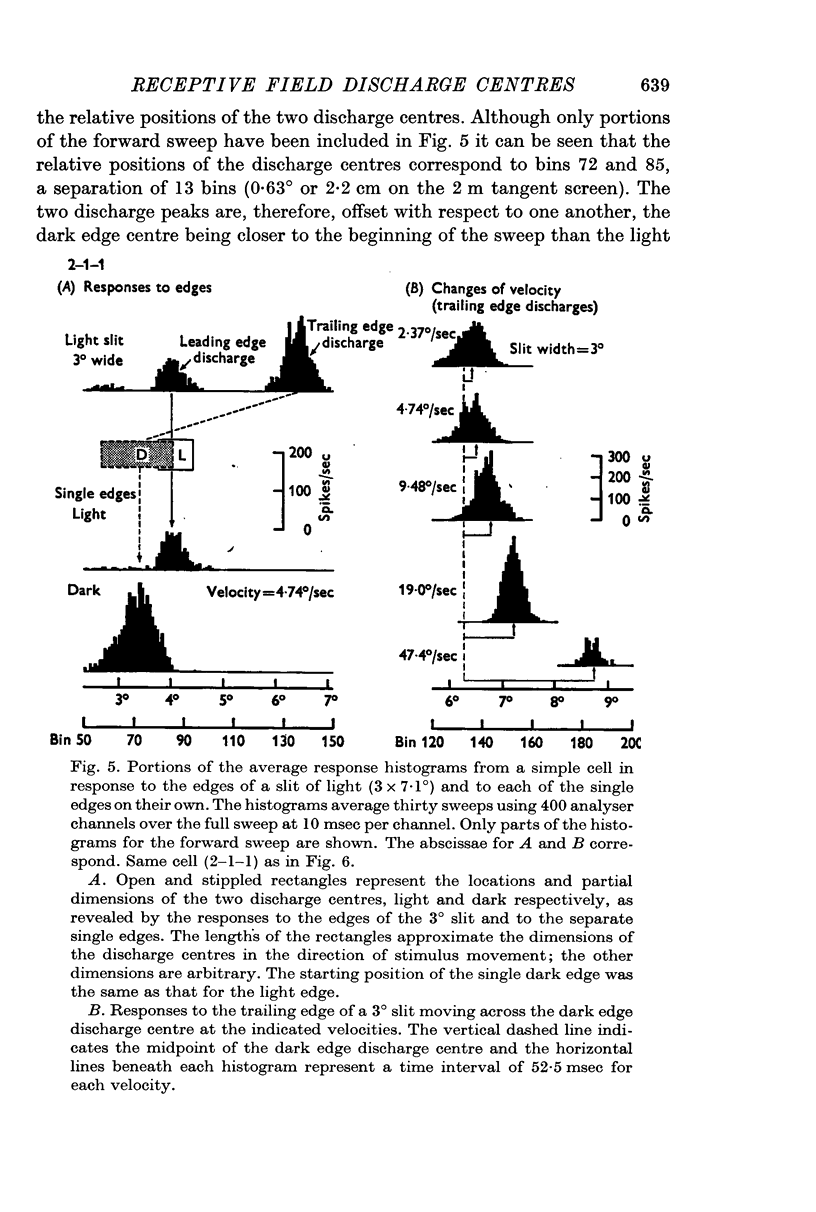
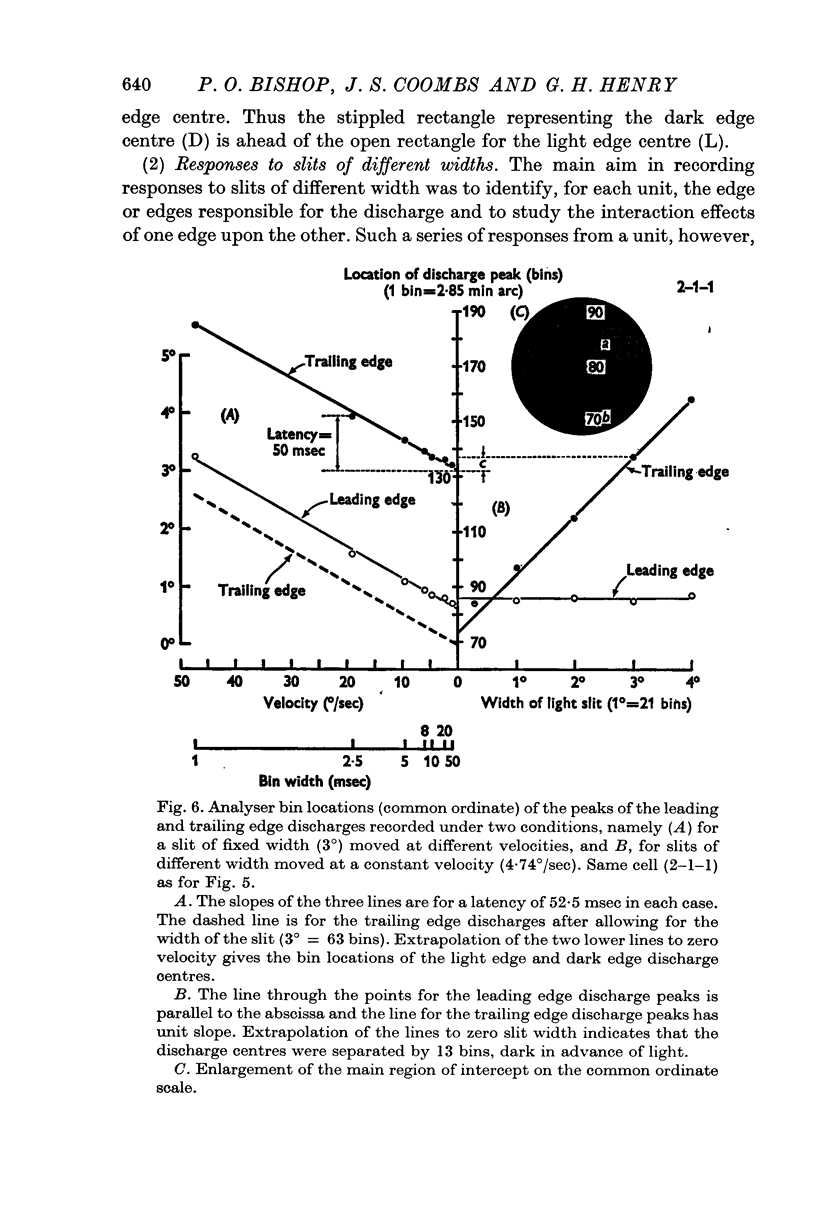
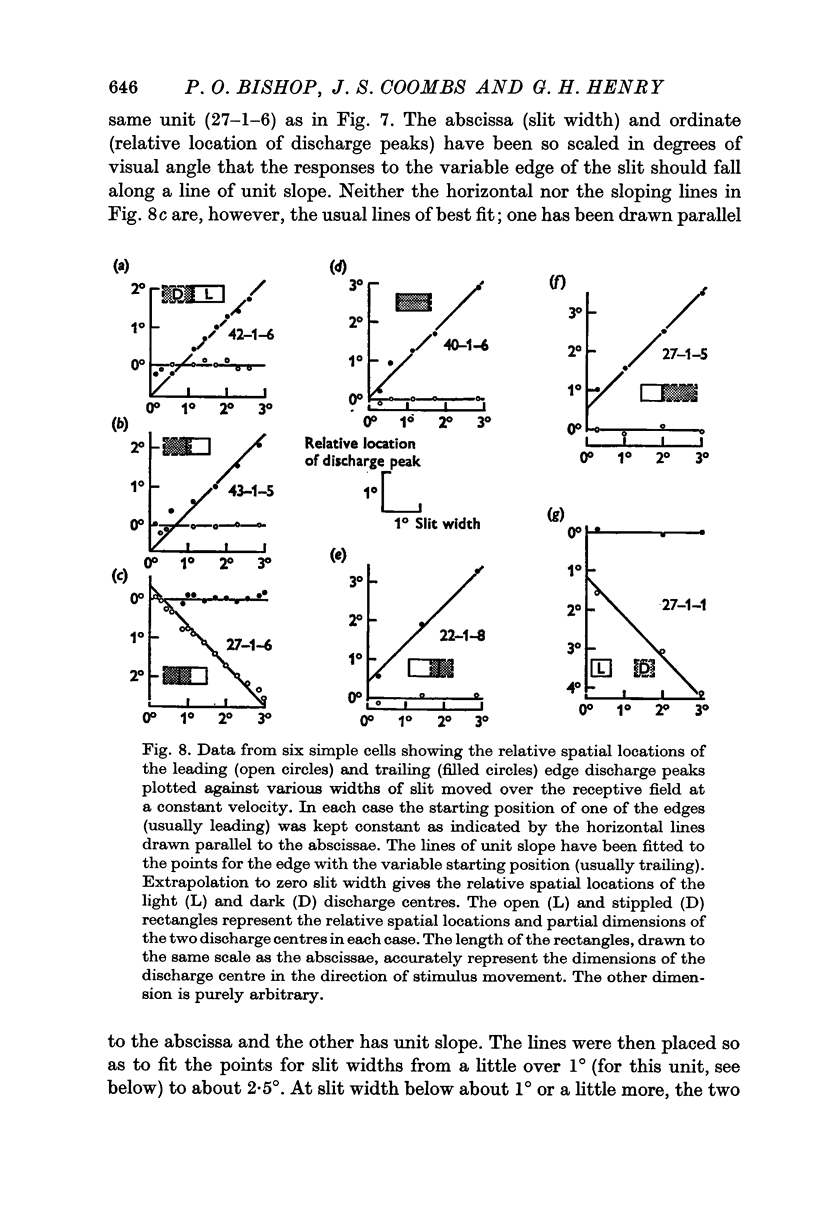
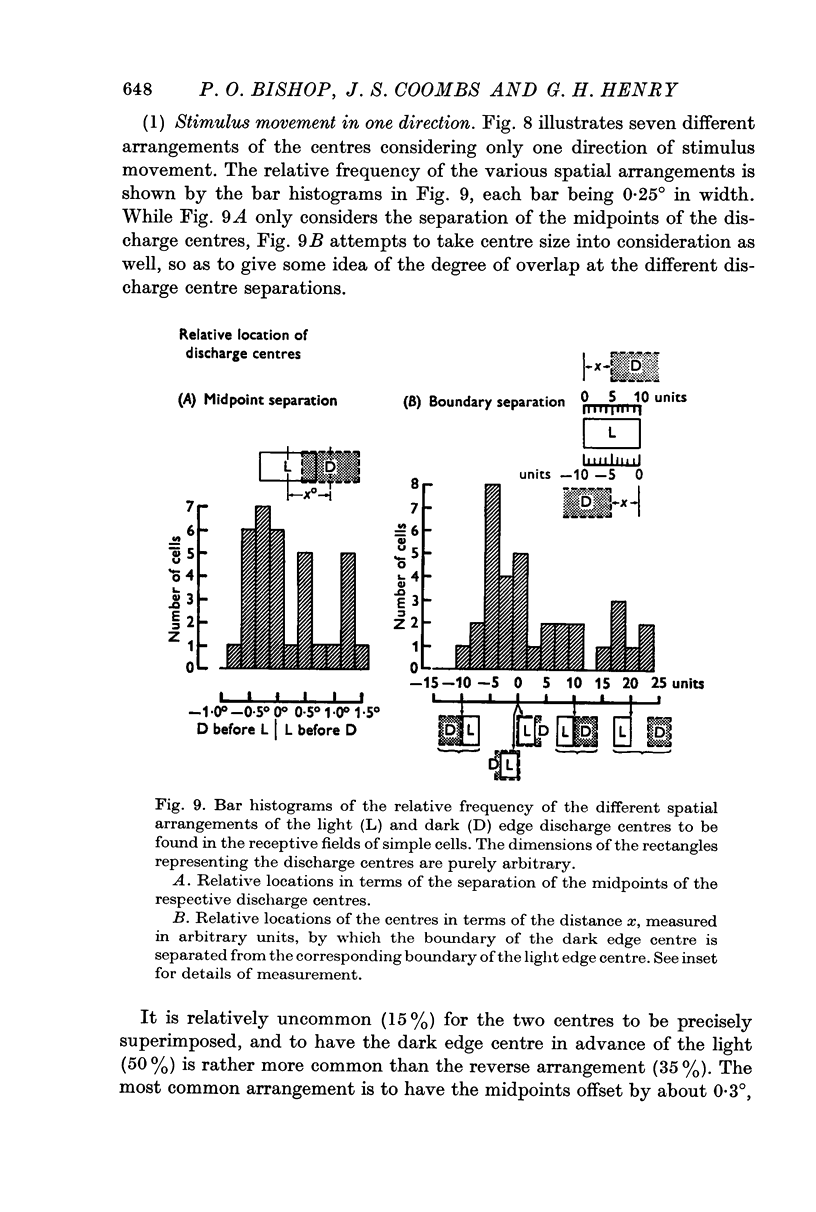
5. Cells responding in one direction with a unimodal average response histogram may be responding to both edges, the two responses being concealed in the one discharge peak. The two discharge centres are then either nearly coincident or, more usually, slightly offset with respect to one another. Most commonly the dark edge centre is slightly in advance of the light edge centre.
6. The discharge peaks in the bimodal and multimodal types come from discharge centres that are spatially separate, each centre firing to only one type of edge. In the case of the bimodal type the light edge centre always lies ahead of the dark edge centre.
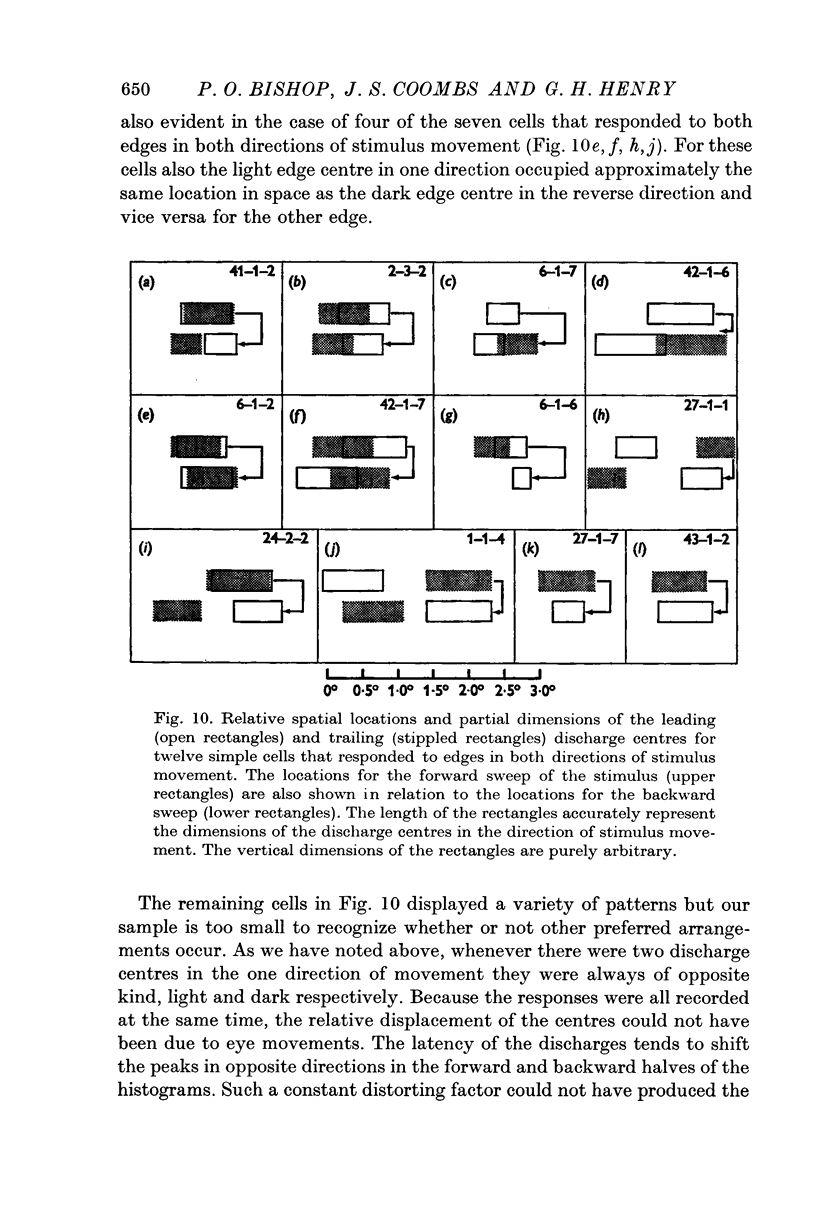
7. When a cell responds to a single edge in both directions of movement, the type of contrast effective in one direction is always the reverse of that in the other. When the cell responded in both directions, whether to one or both edges, most commonly a light edge discharge centre in one direction occupied approximately the same location in space as the dark edge centre in the reverse direction and vice versa for the other edge.
8. Temporal aspects of the discharge of simple cells have been examined by recording the responses to moving slits and single edges over a wide range of velocities.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. O., Coombs J. S., Henry G. H. Interaction effects of visual contours on the discharge frequency of simple striate neurones. J Physiol. 1971 Dec;219(3):659–687. doi: 10.1113/jphysiol.1971.sp009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O., Ito M. Functional synaptic organization of primary visual cortex neurones in the cat. Exp Brain Res. 1968;6(4):324–352. doi: 10.1007/BF00233183. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua D. E., Bishop P. O. Binocular single vision and depth discrimination. Receptive field disparities for central and peripheral vision and binocular interaction on peripheral single units in cat striate cortex. Exp Brain Res. 1970;10(4):389–416. doi: 10.1007/BF02324766. [DOI] [PubMed] [Google Scholar]
- Kinston W. J., Vadas M. A., Bishop P. O. Multiple projection of the visual field to the medical portion of the dorsal lateral geniculate nucleus and the adjacent nuclei of the thalamus of the cat. J Comp Neurol. 1969 Jul;136(3):295–315. doi: 10.1002/cne.901360304. [DOI] [PubMed] [Google Scholar]
- LI C. L., ORTIZ-GALVIN A., CHOU S. N., HOWARD S. Y. Cortical intracellular potentials in response to stimulation to lateral geniculate body. J Neurophysiol. 1960 Nov;23:592–601. doi: 10.1152/jn.1960.23.6.592. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Konishi M., Creutzfeldt O. D. Postsynaptic potentials in the cat's visual cortex following electrical stimulation of afferent pathways. Exp Brain Res. 1966;1(3):272–283. doi: 10.1007/BF00234347. [DOI] [PubMed] [Google Scholar]