Abstract
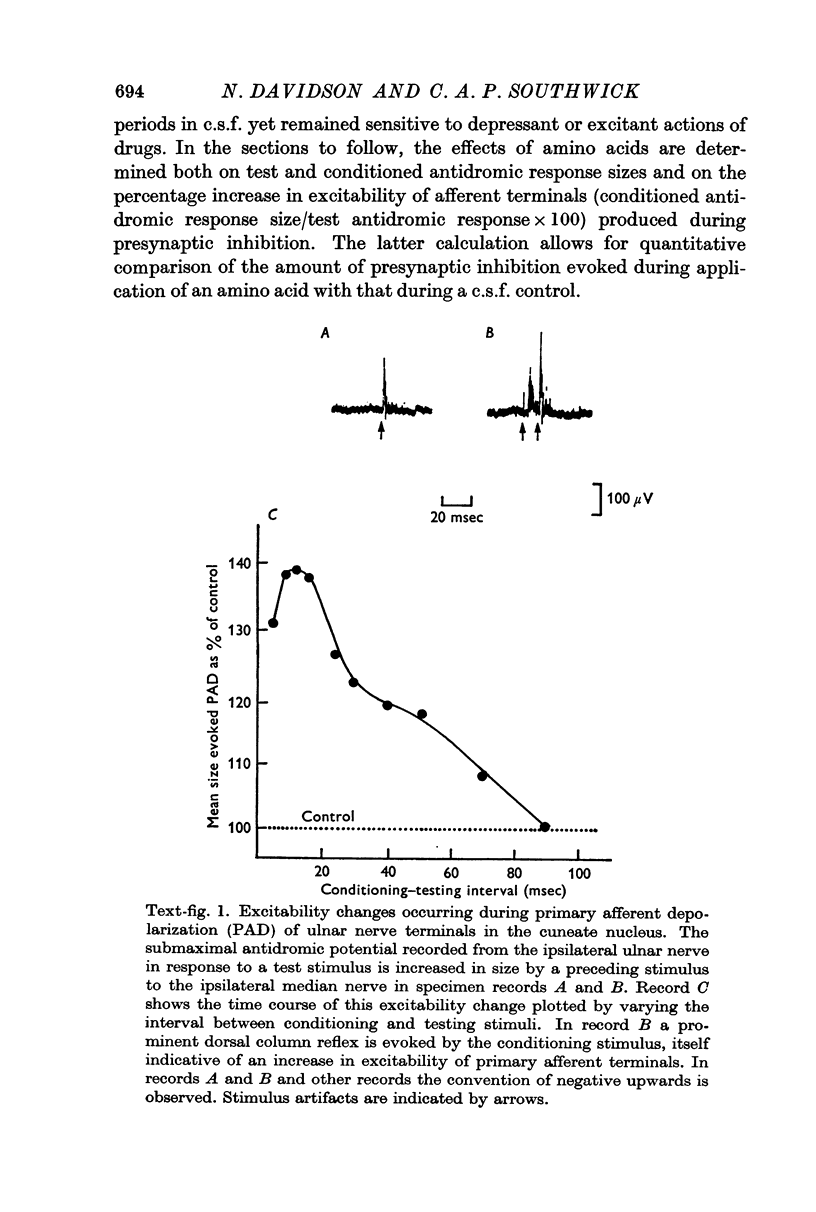
1. Presynaptic inhibition was evoked in the rat cuneate nucleus by a peripheral conditioning stimulus. The dicarboxylic amino acid salts glutamate and aspartate and the neutral amino acids glycine and γ-aminobutyric acid (GABA) were topically applied to a restricted area of the cuneate nucleus and their effects on both resting primary afferent terminal excitability and the increase in excitability of afferent terminals during presynaptic inhibition determined.
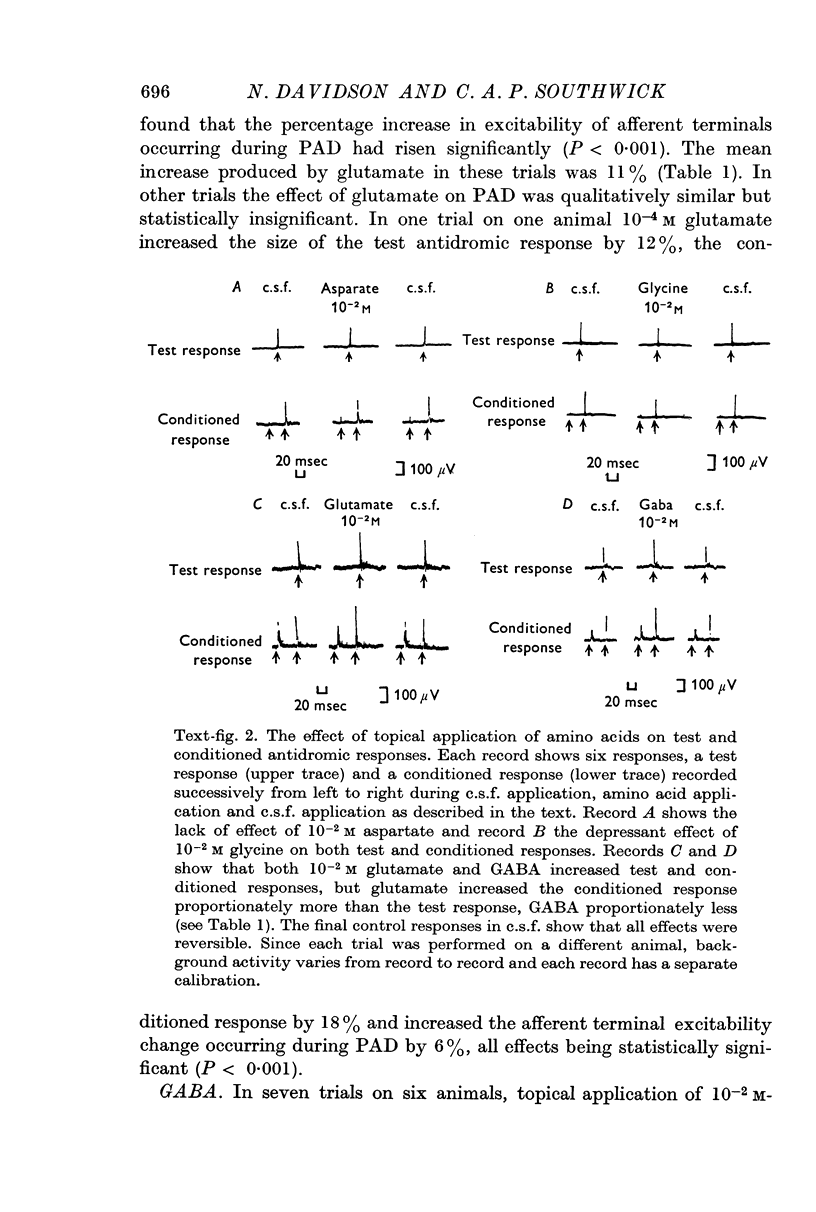
2. Aspartate had no effect on either resting primary afferent terminal excitability or on the increase in excitability during presynaptic inhibition.
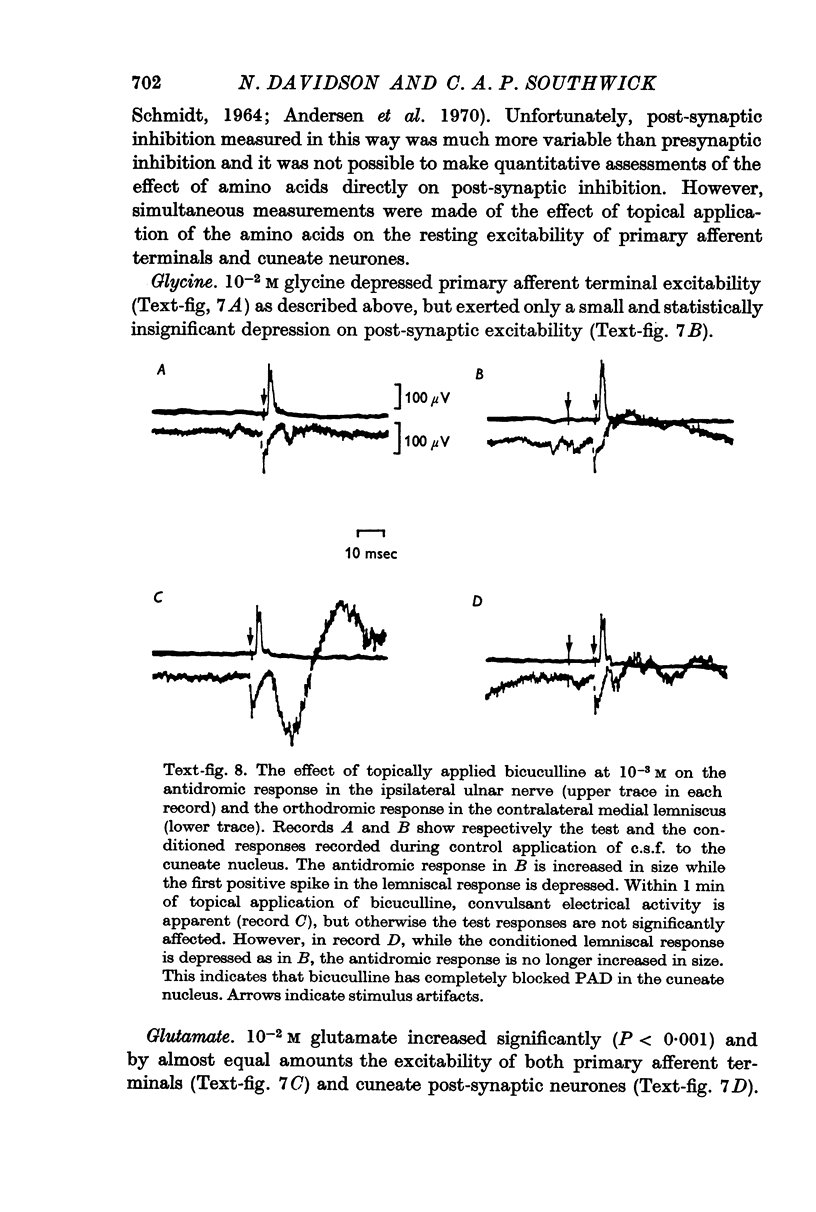
3. Glycine reduced both resting primary afferent terminal excitability and presynaptic inhibition.
4. Glutamate increased both resting primary afferent terminal excitability and presynaptic inhibition while GABA increased resting primary afferent terminal excitability but reduced the increase in excitability during presynaptic inhibition.
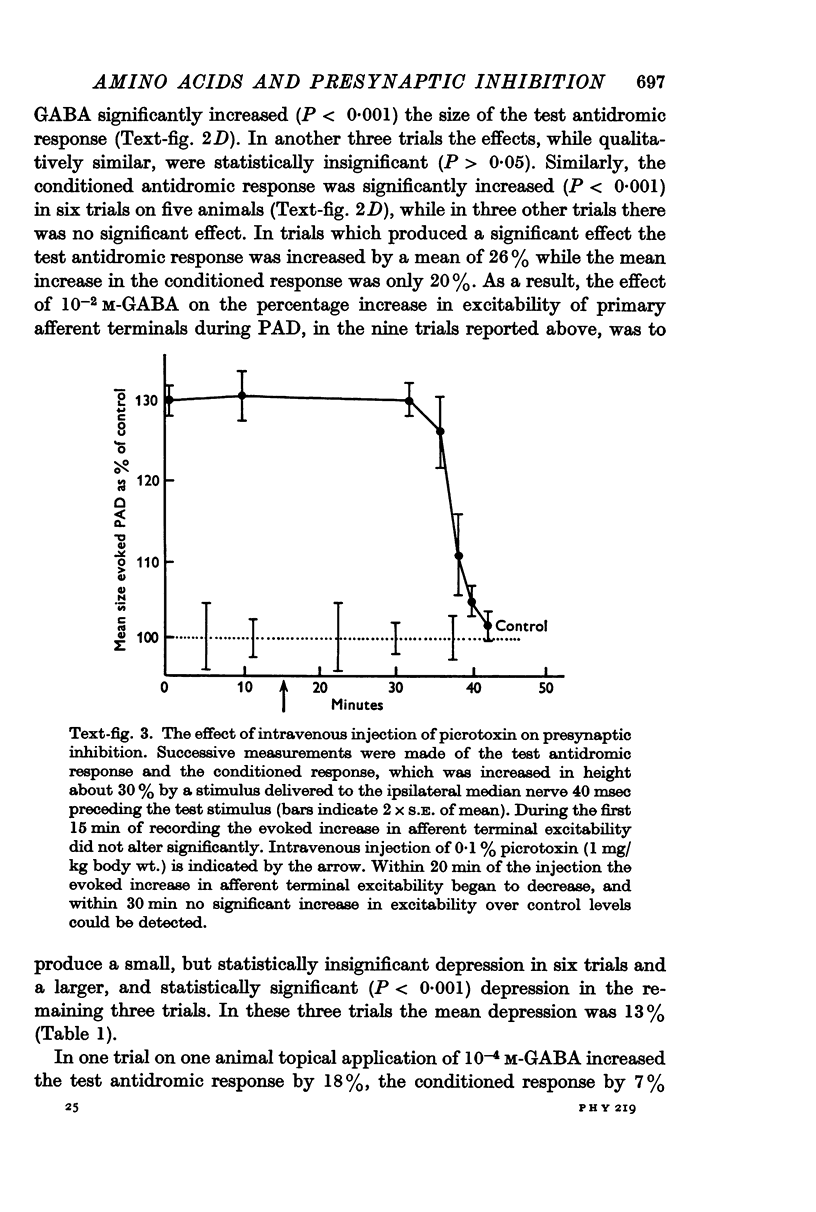
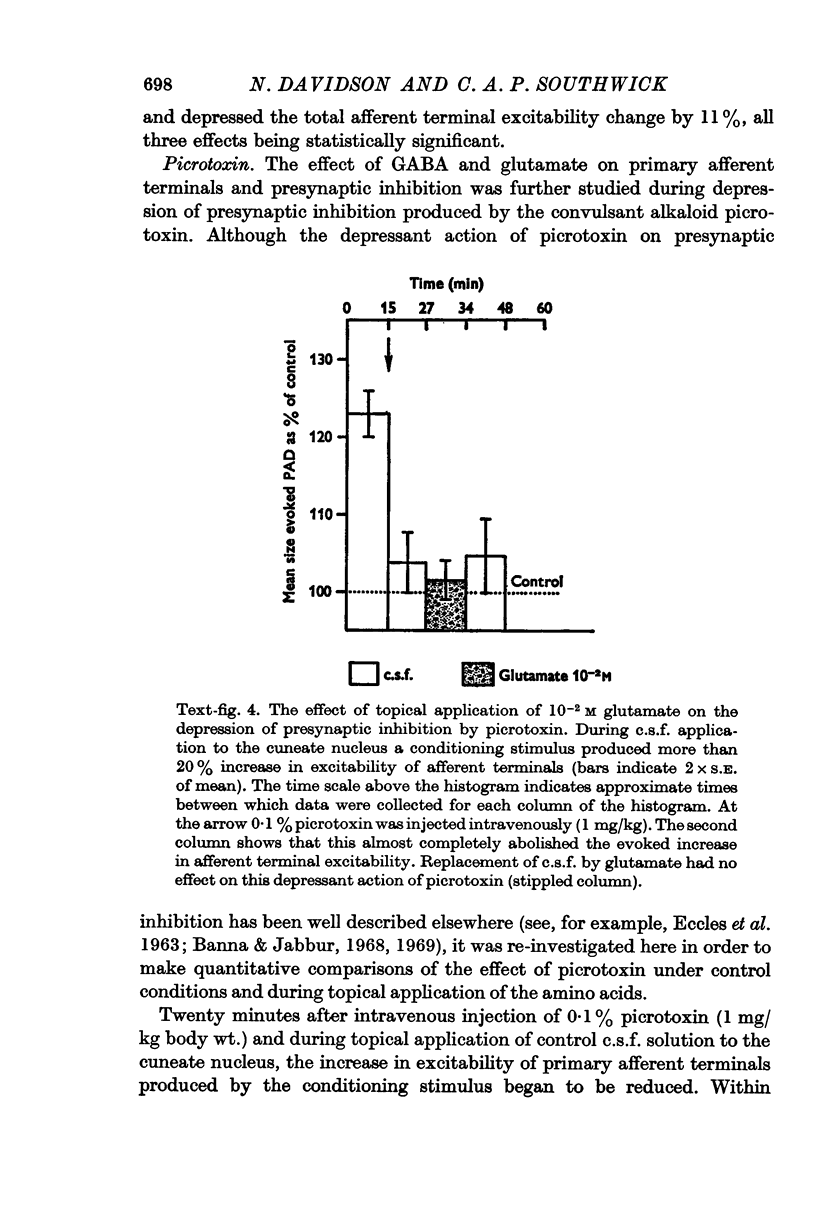
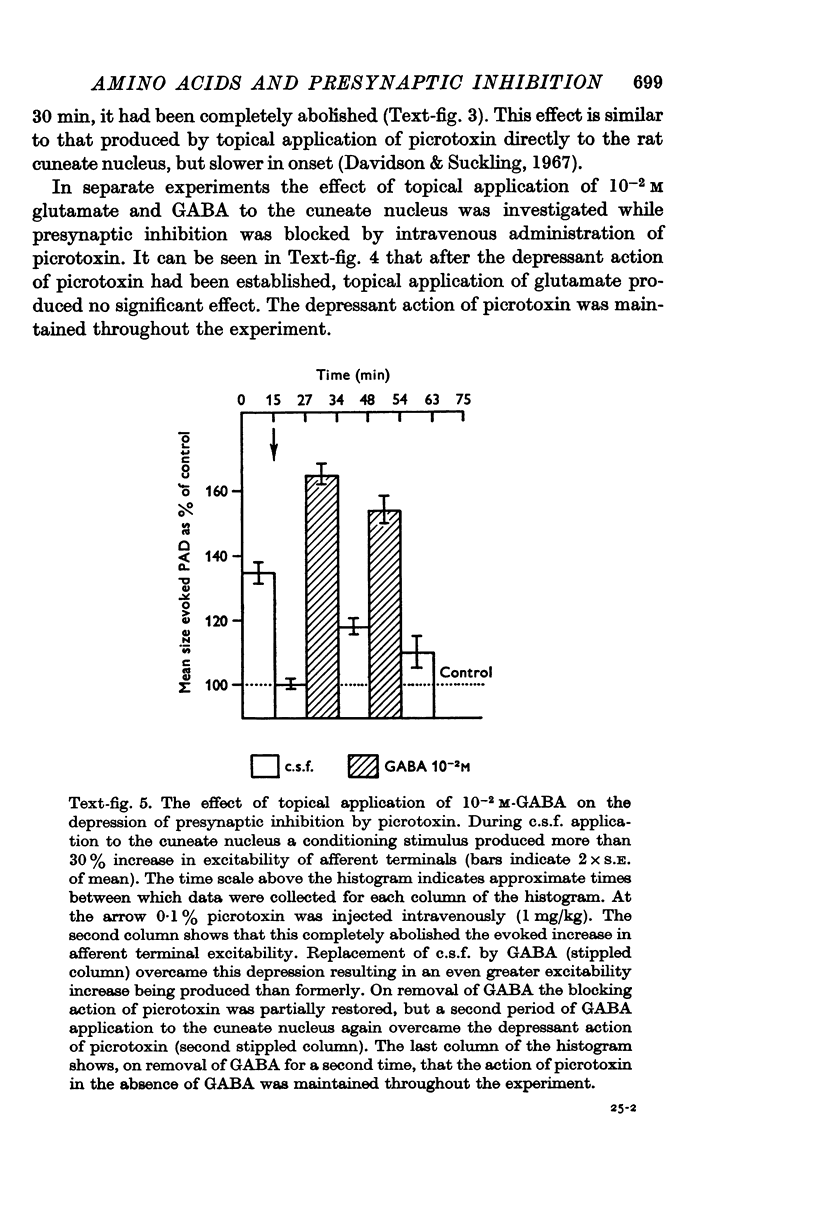
5. The convulsant alkaloids picrotoxin (given intravenously) and bicuculline (topically applied) blocked presynaptic inhibition. The blocking action of picrotoxin was overcome by topical application of GABA but not glutamate.
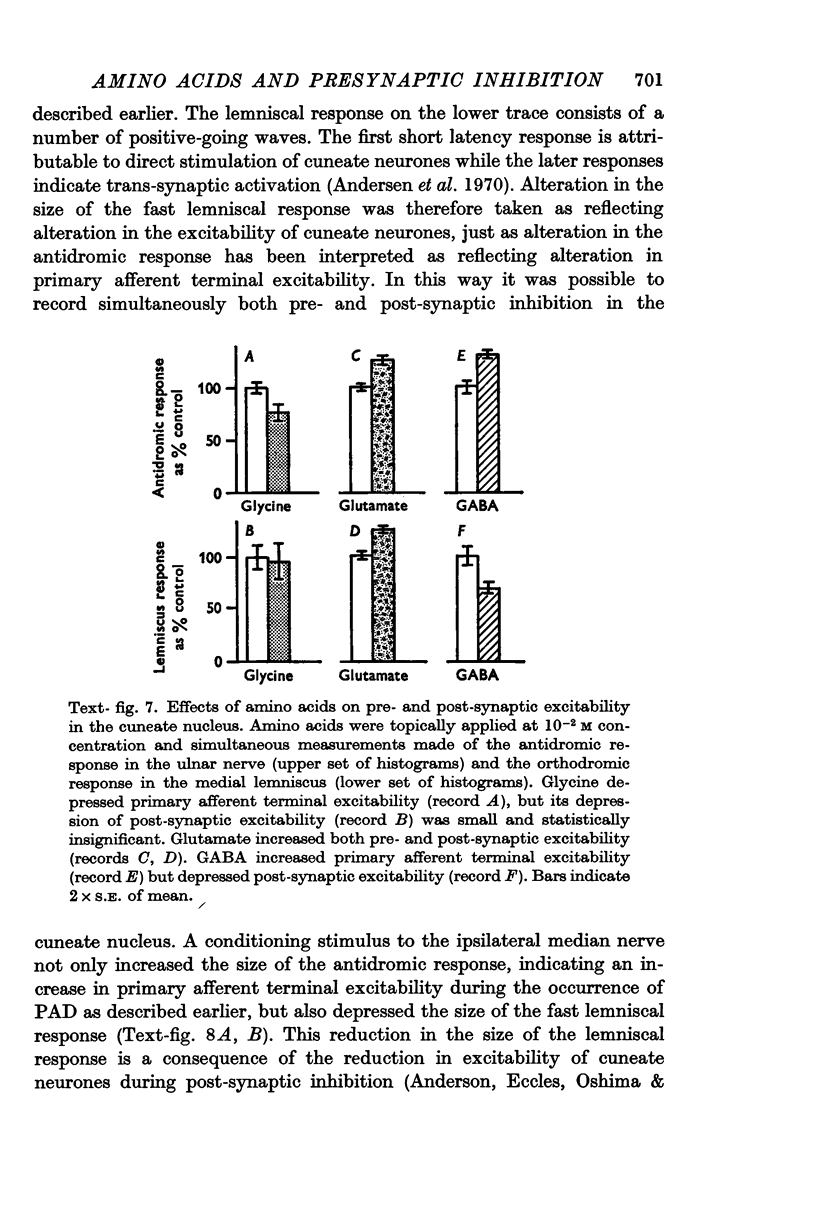
6. Simultaneous measurement of pre- and post-synaptic excitability in the cuneate nucleus showed that while glutamate increased excitability at both sites, GABA increased primary afferent terminal excitability but depressed post-synaptic excitability.
7. It is concluded that glycine and glutamate exert non-specific actions on primary afferent terminals similar to their effects at post-synaptic sites elsewhere in the C.N.S. while GABA depolarizes primary afferent terminals by a specific action at the same receptor site as the presynaptic inhibitory transmitter. The possibility is discussed that the presynaptic inhibitory transmitter in the cuneate nucleus is GABA or a closely related substance.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna N. R., Jabbur S. J. Antagonism of presynaptic inhibition in the cuneate nucleus by picrotoxin. Nature. 1968 Jan 6;217(5123):83–84. doi: 10.1038/217083a0. [DOI] [PubMed] [Google Scholar]
- Banna N. R., Jabbur S. J. Pharmacological studies on inhibition in the cuneate nucleus of the cat. Int J Neuropharmacol. 1969 May;8(3):299–307. doi: 10.1016/0028-3908(69)90051-3. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., FALCK B., FUXE K., HILLARP N. A. CELLULAR LOCALIZATION OF MONOAMINES IN THE SPINAL CORD. Acta Physiol Scand. 1964 Jan-Feb;60:112–119. doi: 10.1111/j.1748-1716.1964.tb02874.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Crawford J. M. Central synaptic transmission--microelectrophoretic studies. Annu Rev Pharmacol. 1969;9:209–240. doi: 10.1146/annurev.pa.09.040169.001233. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. Pharmacological studies upon spinal presynaptic fibres. Exp Brain Res. 1966;1(2):195–204. doi: 10.1007/BF00236871. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Watkins J. C. The pharmacology of amino acids related to gamma-aminobutyric acid. Pharmacol Rev. 1965 Dec;17(4):347–391. [PubMed] [Google Scholar]
- DAWSON G. D., PODACHIN V. P., SCHATZ S. W. Facilitation of cortical responses by competing stimuli. J Physiol. 1963 Apr;166:363–381. doi: 10.1113/jphysiol.1963.sp007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N., Southwick C. A. The effect of topically applied amino acids on primary afferent terminal excitability in the rat cuneate nucleus. J Physiol. 1970 Sep;210(2):172P–173P. [PubMed] [Google Scholar]
- Duggan A. W., McLennan H. Bicuculline and inhibition in the thalamus. Brain Res. 1971 Jan 8;25(1):188–191. doi: 10.1016/0006-8993(71)90579-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961 Nov;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Thaller A. On the interaction of picrotoxin with GABA and glycine in the spinal cord. Brain Res. 1970 Apr 1;19(1):151–154. doi: 10.1016/0006-8993(70)90244-1. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. A morphological basis for pre-synaptic inhibition? Nature. 1962 Jan 6;193:82–83. doi: 10.1038/193082a0. [DOI] [PubMed] [Google Scholar]
- Galindo A. GABA-picrotoxin interaction in the mammalian central nervous system. Brain Res. 1969 Aug;14(3):763–767. doi: 10.1016/0006-8993(69)90220-0. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Aprison M. H. Distribution of some enzymes associated with the metabolism of glutamate, aspartate, gamma-aminobutyrate and glutamine in cat spinal cord. J Neurochem. 1969 Apr;16(4):559–566. doi: 10.1111/j.1471-4159.1969.tb06855.x. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Shank R. P., Werman R., Aprison M. H. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967 Apr;14(4):465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Malcolm J. L., Saraiva P., Spear P. J. Cholinergic and adrenergic inhibition in the rat cerebral cortex. Int J Neuropharmacol. 1967 Nov;6(6):509–527. doi: 10.1016/0028-3908(67)90051-2. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Subcellular distribution of endogenous and (3H) gamma-aminobutyric acid in rat cerebral cortex. J Neurochem. 1969 Aug;16(8):1245–1252. doi: 10.1111/j.1471-4159.1969.tb05972.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- Walberg F. Axoaxonic contacts in the cuneate nucleus, probable basis for presynaptic depolarization. Exp Neurol. 1965 Oct;13(2):218–231. doi: 10.1016/0014-4886(65)90111-1. [DOI] [PubMed] [Google Scholar]



